Biomedical Engineering Reference
In-Depth Information
FIGURE 2.1
Schematic diagram of a bilayer lipid membrane.
FIGURE 2.2
Schematic diagram of a membrane with a hydrophobic pore.
Figure 2.1 will deform to create a small pore, which at this stage is referred
to as a hydrophobic pore, such as the one shown in Figure 2.2. The pore is
hydrophobic because the fatty hydrophobic chains are exposed. This is not a
thermodynamically preferred configuration, and the pore is not stable.
There are substantial experimental data that show that lipid bilayers expe-
rience electrical breakdown when the transmembrane voltage exceeds some
threshold, usually around 200 mV. This may occur after as little as 1
sec, or
up to several milliseconds, depending on different attributes of the membrane
and the environment (Chen et al. 2006). The fact that this initial step can be
detected in both artificial planar bilayer lipid membranes as well as the cell's
membrane indicates that this phenomenon does not depend on some active
processes in the cell and cannot be attributed to unique components of the
cell membrane such as ion channels or other membrane proteins. This does
not mean that such elements do not play a role in the overall electropora-
tion process. In fact, many experiments suggest they do, but the fundamental
phenomenon does not depend on them.
In the second stage, the pores expand as the excitation pulse continues
(Prausnitz et al. 1995). Two simultaneous processes occur. The existing pores
expand in size, increasing the initial radius from less than 1 nm to several
nanometers up to 100 nm. At the same time, new pores continue to form in
those parts of the membrane that are above the electroporation voltage thresh-
old. The bilayer membrane becomes leaky, and significant molecular transport
occurs. This is a complicated scenario since the increase in permeability also
has a great effect on the transmembrane potential. The increase in membrane
conductivity lowers the transmembrane potential, which has an attenuating
effect on the process itself. When the radius of a pore increases, it turns from
a hydrophobic into a hydrophilic pore, such as the one depicted in Figure 2.3.
This kind of pore is much more stable than the hydrophobic pore that was first
created. Molecular dynamics simulations (Tieleman 2004; Tarek 2005) have
µ


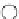
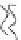
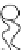







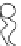

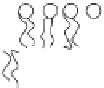
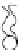
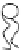
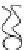
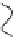


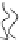
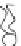
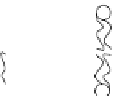

Search WWH ::

Custom Search