Biomedical Engineering Reference
In-Depth Information
Voltage vs. Ag/AgCl (mV)
-218
-0.35
200
-53
-310
-456
47
297
6.0
d(
mass
)/d
t
d(
-0.30
∆
)/d
t
5.5
mass
-0.25
5.0
-0.20
Ψ
4.5
-0.15
current
-0.10
4.0
-0.05
3.5
d(
Ψ
)/d
t
0.00
3.0
150
0.05
0
25
50
75
Time (sec)
100
125
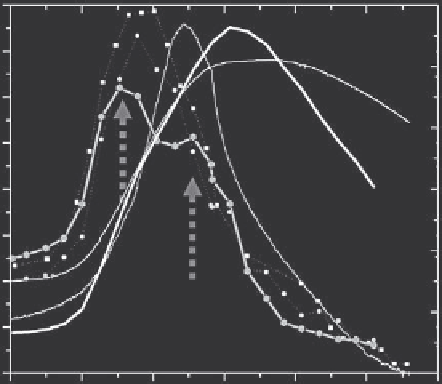
FIGURE 13.14
Correspondence of changes in ellipsometric angles, mass, and current, showing
that the physical process (adsorption) and reduction-induced chemical changes
can be detected in the combined electrochemical microgravimetric ellipsomet-
ric measurement which gives the temporal and spatial (thickness) resolution
to reveal the physical and chemical processes involved in the poly-methylene
green deposition.
AT-, BT-, and rotated Y-cuts, in the crystal structure. The oscillation fre-
quency is related to the crystal thickness and the crystallographic orientation.
Deposition of a thin layer of material on the crystal surface will affect the
oscillation frequency of the crystal in proportion to the mass deposited, a
relationship described by the Sauerbrey's equation (Sauerbrey 1959):
2
f
o
·
ρ
1
/
2
q
µ
1
/
2
q
∆
f
=
−
∆
m/A
·
·
(13.5)
where ∆
f
is the frequency change,
f
o
the resonant frequency of the quartz
crystal, ∆
m
the mass change,
A
the piezoelectrically active crystal surface
area,
ρ
q
the density of quartz,
µ
q
the shear modulus of quartz for AT-cut
crystal (
µ
q
=2
.
947
sec
2
). It should be noted that the Sauerbrey's
equation is strictly applied only to rigid mass on the surface. To perform QCM
in a liquid, a viscosity-related frequency change will be observed (Kanazawa
and Gordon 1985). In such a case, the frequency change should follow:
10
11
g/cm
×
·
∆
f
=
f
3
/
2
o
µ
q
)
1
/
2
·
(
η
l
·
ρ
l
/π
·
ρ
q
·
(13.6)
where
ρ
l
is the density and
η
l
the viscosity of the liquid.

Search WWH ::

Custom Search