Biomedical Engineering Reference
In-Depth Information
CO
2
H
HO
2
C
HN
H
2
N
O
C OH
O
C
NH
B
HO
2
C
NH
2
O
N
HO
2
C
HN
(2)
OH
HO
2
C
O
HN
HO
EDC
PAD
(3)
B
S
(1)
N
OH
N
OH
S
C
O
S
C
N
O
EDG
O
O
Au
Au
O
O
N
O
C
H
N
NH
2
e
-
e
-
Glucose
O
H
N
N
O
HO
2
C
HN
Gox
N
H
O
PQQ
HO
FAD
O
S
S
N
O
Apo-Gox
O
P
P
N
GOx
Gluconic
acid
O
O
-
O
-
O
Au
O
O
N
O
O
O
CHCHOHH
Au
NN
HH H H H
(a)
50
40
30
e
-
Glucose
e
-
H
N
PQQ
FAD
20
S
GOx
10
Gluconic
acid
0
0 0
[Glucose] / mM
40
60
80
Au
(b)
(c)
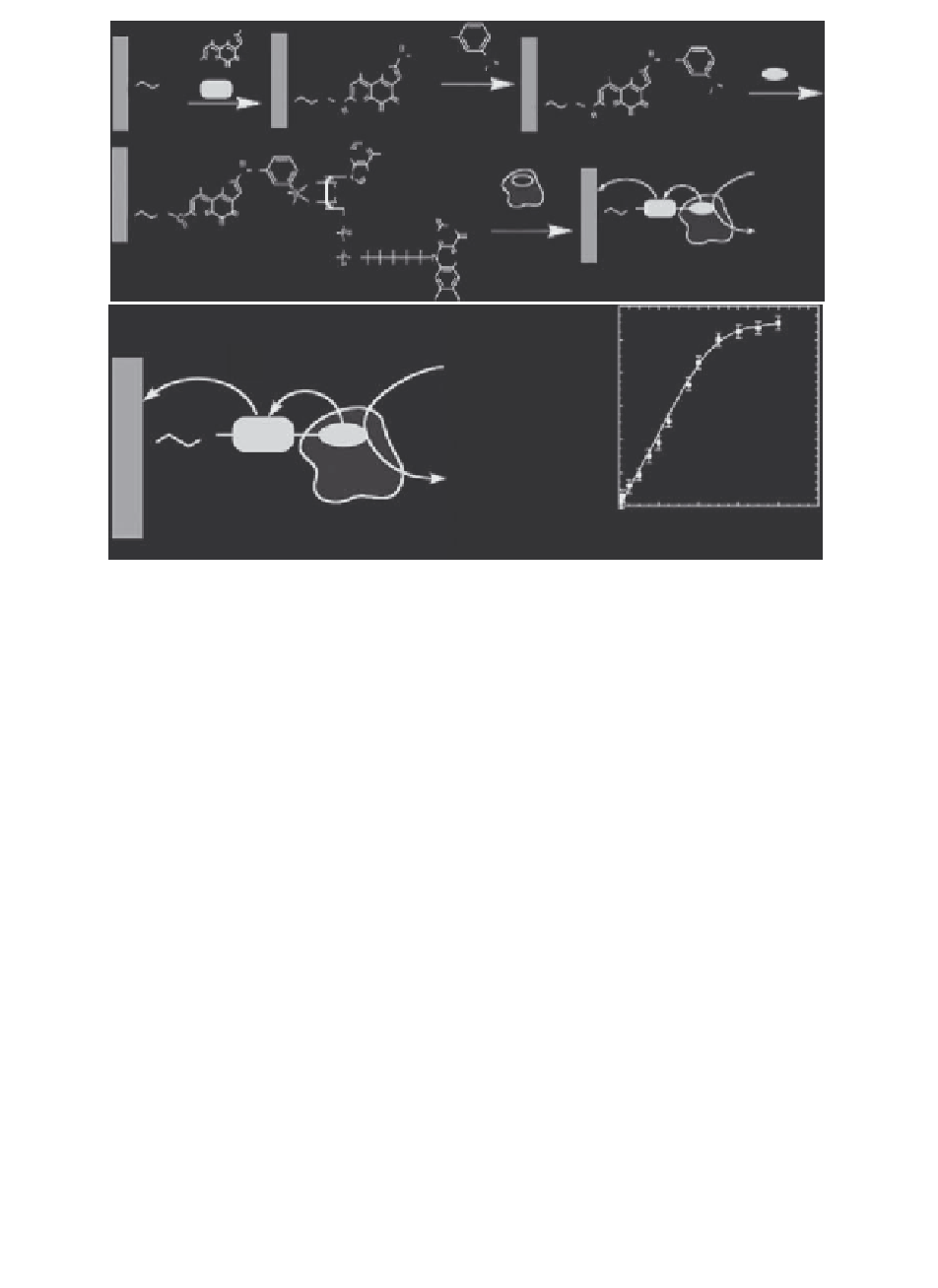
FIGURE 13.6
(a) Assembly of reconstituted GOx bioelectrode, which consists of electroac-
tive relay units, based on functionalized PQQ-FAD and immobilized on Au
electrode. (b) The functionalized GOx electrode that can conduct partial oxi-
dation of glucose to gluconic acid. (c) A calibration plot of the electrocatalytic
current at 0.2 V versus SCE as a function of glucose concentrations.
The advancement of the fuel cells, and more so with biofuel cells, has
increased the need for additional characterization techniques that were not
essential in the case of previous power generating systems. As opposed to
an internal combustion engine in which reactants are mixed before the reac-
tion and thermal energy is generated to create mechanical displacement for
power, a fuel cell generates power when a reactant reacts with a catalyst in
a compartment to realize half of the cell reaction (a reduction or oxidation
as a half-cell reaction) while the other half-cell reaction (in complement to
the reduction or oxidation) occurs at the other compartment, whereas the
electrolyte keeps the electrons flowing in an external circuit to power the
load. Although, this electrochemical route allows electron transfer to achieve
a more ecient chemical energy conversion directly to an electrical one than
Search WWH ::

Custom Search