Biomedical Engineering Reference
In-Depth Information
40
with binding
without binding
30
0.01 Hz at 6% strain
20
0.1 Hz at 2% strain
10
0
0
1
2
3
4
5
Time (h)
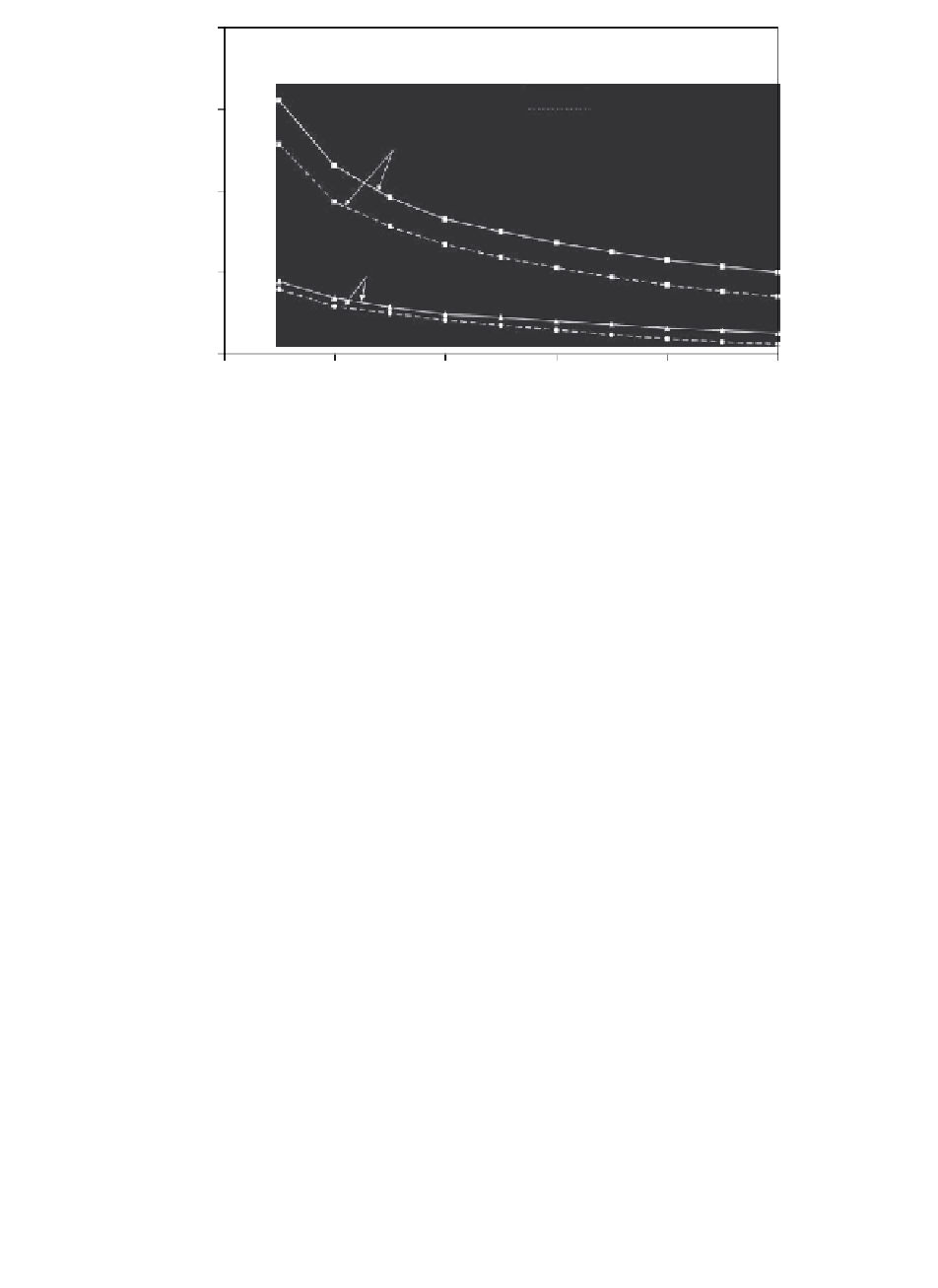
FIGURE 11.11
Comparison of the predicted percent increase in the average free IGF-I uptake
ratio (
R
u
), as a function of time for cases of no binding (interaction) with
IGFBP and with a binding interaction. Note two loading regimes are shown.
In each case (
c
I
0
= 40 nM).
11.4.1.1
Competitive Binding of IGFs to Their IGFBPs
in Cartilage
IGF-I and -II are important stimuli for cartilage ECM synthesis and assembly
[63]. The content of IGFs and IGFBPs in cartilage may vary under various
conditions. In diseased cartilage (e.g., OA and rheumatoid arthritis) the level
of IGF-I in arthritic synovial fluid is found to be increased relative to normal
cartilage, but no change is recorded in the level of IGF-II [62]. IGFBP-3 is
the most abundant binding in human cartilage and significant elevation of
its content was observed in OA cartilage [70]. IGFBPs in cartilage also vary
between species. For example, high content of IGFBP-6 has been identified in
bovine cartilage [29], while IGFBP-6 was too little to detect in human cartilage
[70]. All these changes may influence IGF uptake and ultimately influence
cartilage homeostasis. The effect of biological changes (e.g., IGF concentration
in synovial fluid) can be investigated through parametric studies.
In vivo
, IGF-I and -II competitively bind to a family of at least six IGF-
BPs simultaneously with differential anities [65, 71-73] (Figure 11.12), and
therefore potentially provides a mechanism for modifying the bioavailability
of IGFs to chondrocytes. Measurements of the interaction kinetics between
IGFs and their binding proteins in solutions has revealed that IGFBPs 1-5
have a similar binding preference for IGF-I and -II (although IGFBP-2 has
a slight IGF-II binding preference), whereas the IGFBP-6 differs from other
Search WWH ::

Custom Search