Biomedical Engineering Reference
In-Depth Information
35
30
25
0.01 Hz at 6% strain
20
15
0.1 Hz at 2% strain
0.01 Hz at 2% strain
10
5
0
0
1
2
3
4
5
Time (h)
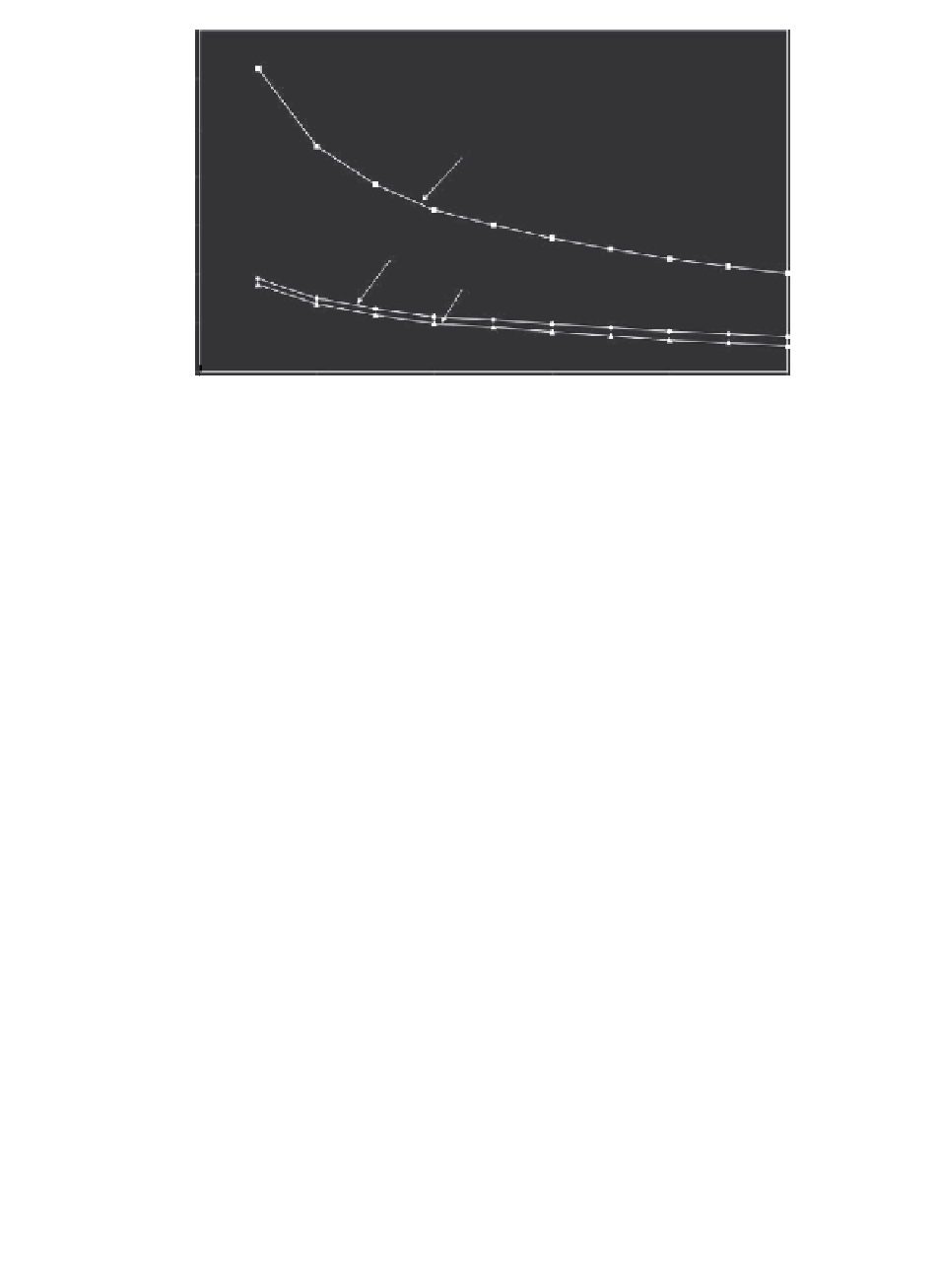
FIGURE 11.4
Predictions of the percent increase (in comparison to case of free diffusion) in
the spatially averaged IGF-I uptake due to cyclic loading. Prediction based
on model presented in Section 11.2.2 using data contained in Table 11.1.
[36] developed a theoretical model based on the theory of porous media to
quantify the effect of cyclic loading on nutrient transport in articular cartilage.
Dynamic loading with 0.01-1 Hz and 0%-20% strain were investigated. The
numerical outcomes provocatively suggested that cyclic loading can actually
concentrate solute inside a cartilage in a variety of cases.
By incorporating strain-dependent diffusion coecient and hydraulic per-
meability into a porous media transport model, Zhang and Szeri [40] showed
that dynamic loading can enhance solute transport in the surface layer more
than in deeper layers, and the beneficial effect is more obvious for large
molecules, even in deeper layers.
However, a recent computational study by Gardiner et al. [28], demon-
strated that any enhancement of solute uptake is strongly time dependent
(see Figure 11.4). More specifically, enhancement only occurs when con-
centration gradients are large and are colocalized to high Darcy veloci-
ties. As high Darcy velocities occur only for high-frequency deformations
or high-strain amplitudes and near the cartilage-solute bath interface (see
Figure 11.5 Darcy velocity), colocalization of large Darcy velocity and high-
solute gradients only occurs in the initial stages of solute uptake into a solute-
depleted cartilage. When these conditions are no longer met, that is, longer
timescales of several hours or more, dynamic loading has negligible effect on
IGF-I transport.













Search WWH ::

Custom Search