Biomedical Engineering Reference
In-Depth Information
(a)
O-H
21
112
PO
4
HPO
4
PO
4
A
O-H
v
1
v
4
v
3
300
xrd
002
CO
3
v
3
CO
3
26
30
34
v
2
B
202
210
102
201
C
(b)
D
Diff
angle
2
θ
(c)
4000
Frequency, cm
-1
3000
2000
1200 1000 800
600
400
b
(d)
26
Diffraction angle, °
30
34 2
θ
a
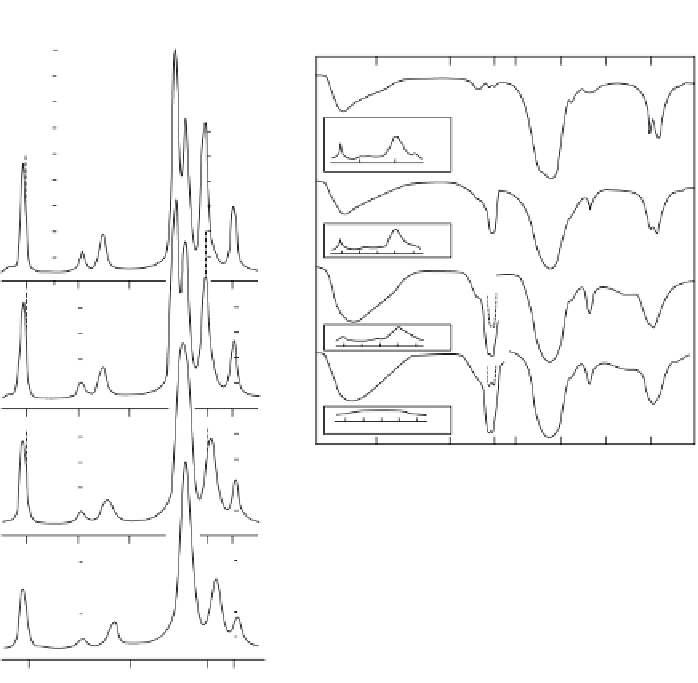
Figure 2.6.
X-ray diffraction profi les of apatites prepared at 95 °C (6a) and 37 °C (6b) with in-
creasing ion concentrations in solution and in the apatite. With apatites prepared at 95 °C (a),
increasing carbonate, broadening of x-ray diffraction peaks indicate decrease in crystallite size
and shift in diffraction peaks indicate changes in lattice parameters caused by CO
3
incorpora-
tion in the apatite. At 37 °C, high carbonate concentration promotes the formation of carbon-
ate containing amorphous calcium phosphate, ACP, shown by the absence of diffraction peaks
and lack of resolution in the phosphate band (6bD) [55,59,87,126].
The type (i) and (ii) carbonate confi guration are associated with the conven-
tional type A infrared carbonate absorption bands at 1451 and 1540 cm
− 1
, while
the type (iii) confi guration is associated with carbonate absorption bands at 1506
and 1571 cm
− 1
(Figure 2.4) [22,24,57,83]. The type (iii) confi guration is a high-
pressure feature. On the other hand, Rietvelt X-ray structure analysis using
hydrothermally synthetic carbonate apatite powders indicated that CO
3
planes
are parallel to the
c
- axis in CO
3
- for - PO
4
substitution [44] .

Search WWH ::

Custom Search