Biomedical Engineering Reference
In-Depth Information
20
16
12
8
4
0
20
16
12
8
4
0
CaP film
CaP - SiO2 film
SiO2 film
CaP film
CaP - SiO2 film
SiO2 film
BG42
BG50
Bulk glass
BG55
BG42
BG50
BG55
Glass coatings
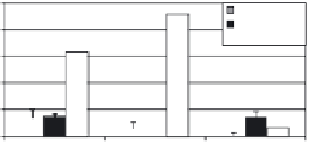
Figure 11.24.
Thickness of CaP, CaP-SiO
2
and silica-rich layers of the bulk and the coatings of
bioactive glasses. From [Liste, 2004b].
bioactivity process explained previously. When the silica content of the glass
decreases, the thickness of the CaP-rich layer increases and, therefore, the glass
bioactivity increases due to the presence of the network modifi ers.
The coatings grown by PLD show a similar behaviour to the bulk glasses but
two important differences can be observed: a decrease in the layer thickness and
a shifting of the
in vitro
bioactivity behaviour with respect to the bulk glass. It
should be noted that a similar bioactivity grade was found for the bulk BG50 and
the coating BG42.
This phenomenon corroborates that the growth of thin fi lm bioactive materi-
als should be carefully controlled because their composition and bonding confi g-
uration play an important role in their bioactivity, which is a key factor for the
development of biomedical products with an adequate biological response.
11.5 BIOCOMPATIBILITY STUDIES FOR MEDICAL APPLICATIONS
11.5.1
In Vitro
Cytotoxicity Test
Testing for cytotoxicity is a good fi rst step towards ensuring the biocompatibility
of this innovative product for its application in biomedical devices. A negative
result indicates that the material is either free of harmful extractables or contains
a quantity of them insuffi cient to cause serious effects in isolated cells under exag-
gerated conditions. However, it is certainly not, on its own merit, evidence that a
material can be considered biocompatible; it is simply a fi rst step. On the other
hand, a positive cytotoxicity test result can be taken as an early warning sign that
a material contains one or more extractable substances that could be of clinical
importance. In such cases, further investigation is required to determine the utility
of the material.
The biological response of the human osteoblast-like cell line MG-63 (ATCC
number CRL 1427) to different biomorphic SiC types and to SiC ceramics coated
with bioactive materials has been determined [de Carlos, 2006; Borrajo, 2006].
The cells were regularly grown in EMEM (EBSS) culture medium supplemented
with 2 mM glutamine, 1% non-essential amino acids and 10% foetal bovine serum
(FBS), at 37 °C in a humidifi ed atmosphere with 5% of CO
2
.






Search WWH ::

Custom Search