Biomedical Engineering Reference
In-Depth Information
8
8
6
6
4
4
μ
m
μ
m
2
8
2
8
6
6
4
4
0
2
0
2
0
m
(a) 2.12
μ
m grain size titania
μ
μ
m
0
(b) 32
nm grain size titania
0.8
0.8
0.6
0.6
μ
m
0.4
0.4
μ
m
0.2
0.2
0.8
0.8
0.6
0.4
0
0.6
0
0.4
0.2
0.2
0
μ
m
μ
m
0
(c) 177
nm grain size alumina
(d) 23
nm grain size alumina
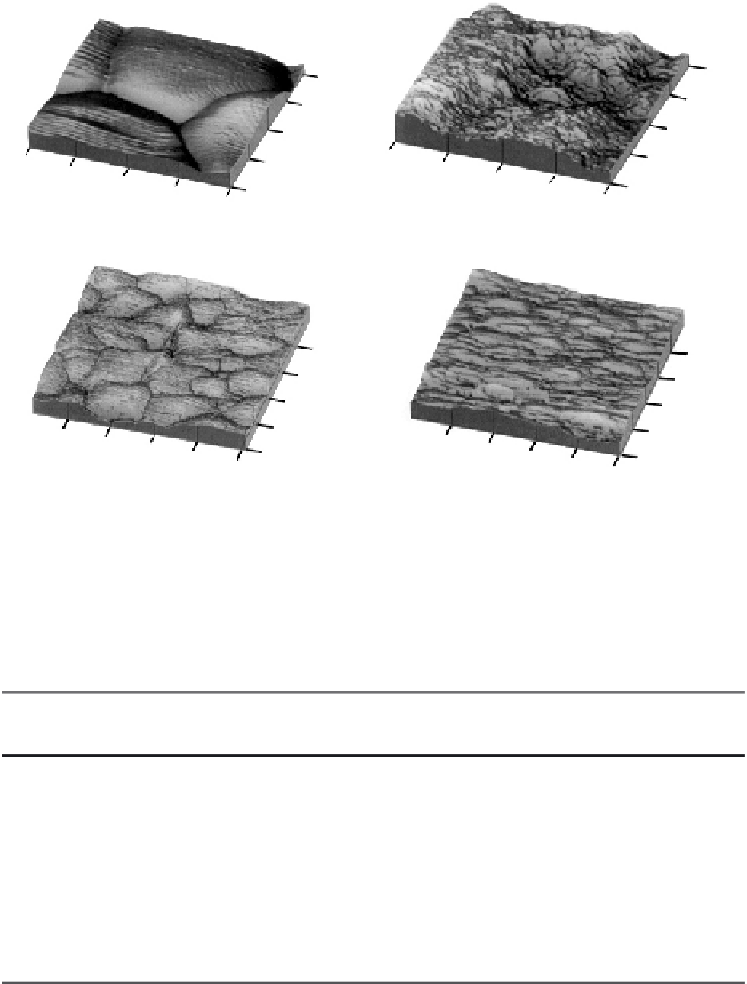
Figure 7.3.
Atomic force microscopy images of titania and alumina. (a) and (c) represent
conventional titania and alumina, respectively, and (b) and (d) represent nanophase titania
and alumina, respectively. Adapted from [14].
TABLE 7.1. Nanophase and Conventional Ceramic Structure and Surface Wettability, Data
is From [16]
Pore diameter
(nm)
Surface
roughness (nm)
Contact angle
(degrees)
Material
Porosity (%)
24 nm alumina
4.5
0.69
20
6.4
±
0.7
45 nm alumina
3.4
1.11
19
10.8
±
1.3
167 nm alumina
2.4
2.94
17
18.6
±
0.9
39 nm titania
4.1
0.98
32
2.2
±
0.1
97 nm titania
3.8
1.91
24
18.1
±
3.2
4520 nm titania
3.2
23.3
16
26.8
±
2.8
67 nm HA
1.1
0.66
17
6.1
±
0.5
132 nm HA
1.1
1.98
11
9.2
±
0.4
179 nm HA
1.1
3.10
10
11.5
±
1.1
In order to illustrate the advantages of nanophase ceramics in terms of
surface properties for orthopedic applications, calcium phosphate derivatives are
perfect examples—such as HA, tricalcium phosphate, calcium carbonate and
bioglass which have extensive applications in orthopedic implants [49]. Since
they share a similar crystal structure and chemical composition to natural bone,
Search WWH ::

Custom Search