Biomedical Engineering Reference
In-Depth Information
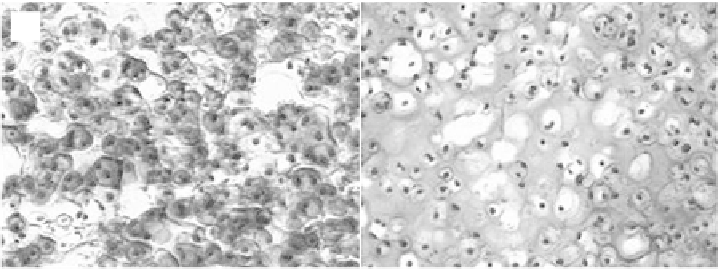
(A)
(B)
Figure 6.1.
Histological analysis of chondrocytes photoencapsulated in PEG hydrogels with
15% macromer after 8 weeks
in vitro
. A. Stained with Safranin-O which stains proteoglycans
red and B. Stained with Masson trichrome which stains collagen blue (
×
200). (See color insert.)
the highest production of collagen II by the encapsulated cells and homogeneous
distribution of glycosamino glycans within the gels using degradable PEG-based
injectable hydrogels compared to non-degradable PEG hydrogels [Bryant and
Anseth, 2002, Bryant et al., 2003]. The feasibility of modulating the macroscopic
properties and degradation behavior of degradable poly(ethylene glycol) hydro-
gels in order to optimize the properties of scaffolds for cartilage tissue engineer-
ing was demonstrated [Bryant et al., 2004; Rice and Anseth, 2004]. By increasing
the macromer concentration, gels with initial compressive moduli of 60-55 kPa
were obtained and incorporation of degradable cross-links into the network
facilitated the diffusion of proteoglycans into the extracellular regions of the
hydrogel. Figure 6.1 is the histological sections of chondrocyte encapsulated PEG
hydrogels with 15% macromer after eight weeks in culture showing the formation
of proteoglycans and collagen by the encapsulated cells [Bryant et al., 2004]. The
study thus demonstrates the feasibility of tailoring the composition of the gels to
control the degradation as well as temporal changes in the gel network structure.
PEG-based injectable photo cross-linkable hydrogels have also been found
to be a potential cell carrier system for neural transplantation. Neural cells cul-
tured within the three-dimensional polymer network created their own cellular
microenvironment to survive, proliferate and differentiate to form neurons and
glia that are electrophysologically responsive to neurotransmitters [Mahoney and
Anseth, 2006]. Also, it has been demonstrated that by changing the degradation
time of the hydrogels, the time-scale over which neural cells extend processes can
be tuned from one to three weeks, since upon degradation gels provide space for
cellular processes to extend through the three dimensional matrix. Figure 6.2
shows the spatial integration of the microtissue fl uorescently labeled with calcein-
AM (green-live) or ethidium bromide (red-dead) in the gel after 16 days in cul-
ture [Mahoney and Anseth, 2006 ].
A recent study demonstrated the minimally-invasive implantation of
PEG hydrogel and subsequent chondrogenic differentiation of encapsulated
Search WWH ::

Custom Search