Biomedical Engineering Reference
In-Depth Information
are low E-modulus and hardness values of polymeric materials. As mentioned
in Table 3.2 the E-modulus of cortical bone is in the range of
17 GPa. Efforts
should be made to develop polymer matrix composite materials, whose E-
modulus and hardness can be raised to more than 8 GPa and 0.5 GPa, respec-
tively. The maximum hardness of cortical bone is nearly
∼
0.5 GPa. With an
increase of E-modulus and hardness, the wear resistance of polymer is expected
to be improved. The development of nanobiocomposites could be useful for such
applications.
Considering their high fracture toughness/strength, metals are an excellent
choice in load-bearing applications. The development of newer alloys will defi -
nitely broaden the area of materials selection. However, many aspects of
in vitro
and
in vivo
evaluation need to be carried out for newer alloys (such as Ti-5Al-2.5
Fe). More research efforts need to be invested to improve the corrosion and wear
resistance of metallic biomaterials inside the human body. The required proper-
ties for hard tissue replacement biomaterials (ceramics, metals and polymers and
their composites) are from a literature review and shown summarized in a sche-
matic diagram in Figure 3.17 .
Future efforts in the development
135
of biocompatibility methodology will
probably be directed towards the development of materials, which will allow nor-
mal differentiation and function of tissues into which the materials are placed. In
predicting areas in which new research will probably occur, two areas should be
mentioned: molecular biology of host tissue in response to materials; and molecu-
lar interactions between biological molecules and synthetic materials or tissue-
material combinations.
∼
Figure 3.17.
The properties of an ideal hard tissue replacement biomaterial are showing in
block diagrams.
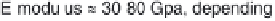



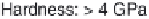





























Search WWH ::

Custom Search