Biomedical Engineering Reference
In-Depth Information
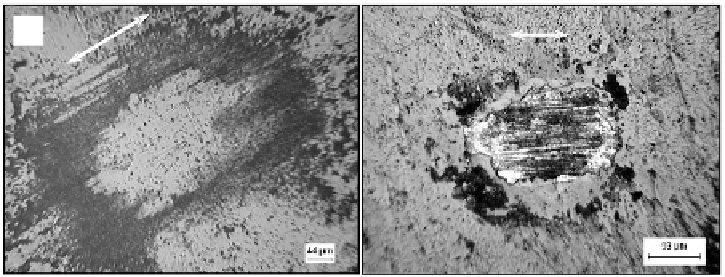
(a)
(b)
Figure 3.16.
Optical microscopy images of the worn surface on DLC coated Co-Cr-Mo surface
illustrating: the mild wear after fretting at 10 N load for 10,000 cycles (a) and the severe wear
after fretting at 10 N load after 100,000 cycles (b). Arrows indicate fretting directions. The
counterbody is bearing steel and the double pointed arrows indicate the fretting. DLC coating
is worn through as seen by the white contrast in (b)
128
.
In a different work, Choubey et al.
128
explored the tribological behavior of
CVD-DLC coated Co-Cr-Mo alloys under fretting contacts. They conducted the
wear experiments on coated materials in Hank's balanced salt solution to assess
the
in vitro
performance in simulated body fl uid (physiological) solution. In SBF
solution, DLC-coated Co-Cr-Mo alloys exhibited low COF of 0.07-0.10, whereas
high COF (0.4) was experienced by uncoated Co-Cr-Mo, under identical fretting
conditions. The wear mechanism was mainly governed by the mild wear with no
signifi cant change in surface morphology in DLC-coated fl at material. The un-
coated Co-Cr-Mo exhibited extensive plastic deformation and delamination in-
duced grain pull out at higher load of 10 N after 10,000 cycles (see Figure 3.16).
The obtained research results indicated the superior tribological performance of
DLC coatings as compared to uncoated Co-Cr-Mo alloys.
In a review article, Hanawa
129
summarized the effect of metal ion release
from the metallic implants
in vivo
. The oxide fi lms, formed on the surface of me-
tallic materials, play an important role and protect the surface to release ions.
Low concentration of dissolved oxygen, inorganic ions, proteins, and cells may
accelerate the metal ion release. Also, the formation and breakdown as well
as regeneration time of oxide layer on metallic implant surface may control the
release rate.
In some specifi c applications, ions/debris can be released from the implants
due to some mechanical actions, like wear and fretting. The behavior of metal ion
release into biofl uid is governed by the electrochemical rule. The released metal
ions do not always combine with biomolecules to casue toxicity, because an active
ion immediately combine with a water molecule or an anion near the ion to form
an oxide, hydroxide, or inorganic salt. It was concluded
129
that there is little
chance that the metal ions can combine with biomolecules to cause cytotoxicity,
allergy, and other biological infl uences.
Search WWH ::

Custom Search