Biomedical Engineering Reference
In-Depth Information
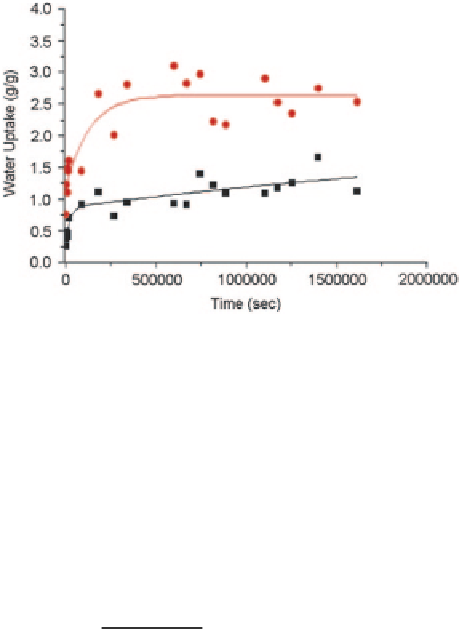
Fig. 3.10
Comparison of
water uptake between PHBV
scaffolds (
black square
)
and 10 % HA in PHBV
composite scaffolds (
red
circle
) at 37 °C (Sultana and
Khan
2012
)
W
i
−
W
f
(3.2)
W ei ght Loss
(
%
) =
×
100
W
i
where; W
i
and W
f
are specimen weights before and after soaking in PBS.
In order to calculate water uptake, pre-weighed scaffolds specimens and thin
films were removed periodically, washed with distilled water, blotted dry on fil-
ter paper in order to remove excess water, weighed and returned to the PBS. The
water uptake was calculated using the following equation:
Water U ptake
=
(
W
w
−
W
d
)
W
d
(3.3)
×
100
where; W
d
and W
w
are specimen weights before and after soaking in PBS.
The compressive mechanical properties of the samples were determined prior
to placement of the samples in the physiological solution (time zero). The samples
were removed at each specified time period throughout the duration of the test and
retest using the originally selected mechanical test methods and conditions. The
morphologies of the as fabricated and degraded composite scaffolds were studied
with a scanning electron microscopy (SEM; Stereoscan 440, Cambridge) at 12 kV.
All the scaffolds exhibited open porous morphology. For the scaffolds fabri-
cated from 10 % (w/v) solution had uniform pore structures which had the pore
sizes ranging from 70 to 600
μ
m with the average pore size of 297
μ
m. On the
other hand, nHA/PHBV composite scaffolds had pore size range of 50-450
μ
m.
The average pore size of nHA/PHBV composite scaffold was 210
μ
m.
Figure
3.10
shows the comparison of the water uptake curves between PHBV
polymer scaffold and 10 % nHA in PHBV composite scaffolds at 37 °C. It was
observed that the initial water uptake of HA incorporated composite scaffold was
much higher than that of polymer scaffold. Approximate equilibrium reached
almost at the same immersion time for both of the scaffolds.
Molecular weights of PHBV scaffolds immersed in the PBS solution at 37 °C
were measured at different time points. It was observed that the molecular weight

Search WWH ::

Custom Search