Biomedical Engineering Reference
In-Depth Information
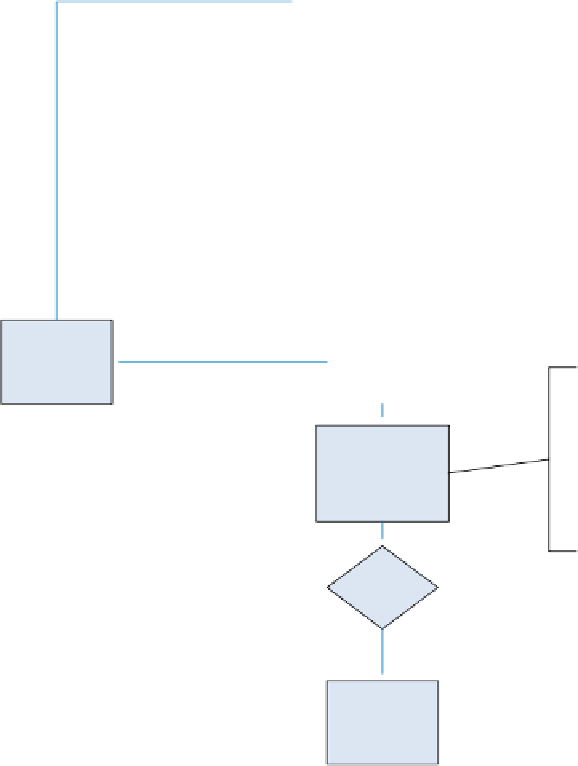
START: Change
initiator submits
change request
Initial review
by change
leader
First risk
assessment (initial
screening)
N
In scope?
CR not required
Y
Y
Return to
change initiator
for more
information
More
information
req'd?
Second risk
assessment
(review impact to
existing product
risk assessment
document or
prepare a new
one)
N
Detailed review
by change
review team
Y
More
information
req'd?
N
Change
approved-OK
to implement
Implement
according to
plan
Change
review team
identifies
actions needed
to rectify
issues
N
Y
Deviations from
plan?
Close out-
done
Figure 13.1
The change control process. (
See insert for color representation of the
figure
.)



































Search WWH ::

Custom Search