Biomedical Engineering Reference
In-Depth Information
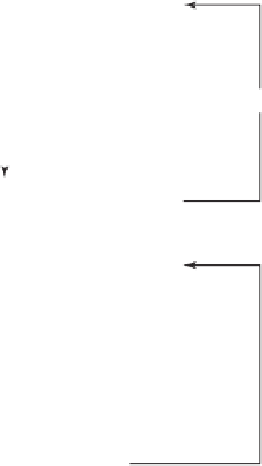
Initiate
quality risk management process
Risk assessment
Risk identification
Risk analysis
Risk evaluation
Unacceptable
Risk control
Risk reduction
Risk acceptance
Output/result of the
quality risk management process
Risk review
Review events
Figure 1.1
Overview of a typical quality risk management process.
• improve the process
• provide information needed to help make decisions
Risk management approaches should
• focus on risk to patient safety
• result in improved process understanding
• result in improved process
• be planned, logical, and documented
• add value
• avoid “checklist” approach
• should support and be consistent with the validation program
• should be documented























Search WWH ::

Custom Search