Biomedical Engineering Reference
In-Depth Information
Assess severity of
excursions beyond
specification limits
Assess likelihood
of occurrence of
excursions beyond
specification limits
Assign
criticality to
individual
quality attribute
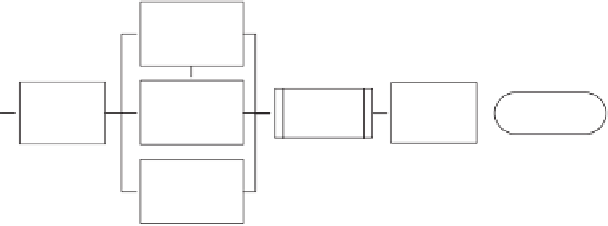
List individual
process
parameters
Create process
map
Assess risk
acceptability
Establish control
strategy
Assess
detectability of
excursions beyond
specification limits
Figure 12.2
Identification of critical process parameters.
evaluation can be based on retrospective information such as general scientific
knowledge, experimental data, and nonconformance results. Prospective eval-
uations can also be performed using the results of designed experiments, and
validation studies. Using this information, the evaluators may identify a subset
of parameters that have the potential to impact product safety and efficacy.
The next step in the process is to assign criticality to the parameters identified.
Criticality is assigned on the basis of three risk factors: severity, occurrence, and
detectability [5].
Severity is evaluated by assessing the impact of a process parameter on product
quality and patient safety. Determine how the parameter would impact a CQA
if it were to exceed its maximum operating range, for example, batch record
limits. In some instances, the maximum operating range for a parameter is well
within the range proved to be acceptable to product quality. In these instances,
an out-of-tolerance parameter would be expected to have minimal impact on
its associated CQA. In other instances, a parameter's maximum operating range
may be very close to its proven acceptable range and excursions have a greater
potential to impact product quality. Thus, its potential impact on product quality
may be very severe.
The next step in assigning criticality to a parameter is to assess the likelihood
that a parameter will exceed its maximum operating range. The likelihood of
exceeding an operating range can be identified through both experimental studies
and practical experience with the same or similar processes. Appropriate engi-
neering controls should be established to insure that processes operate within
specified ranges, thereby reducing the likelihood of an excursion.
Detectability is a factor that describes one's ability to identify an excursion
before it affects patient safety or product quality. Detectability evaluations should
consider both the ability to detect a process excursion and the ability to detect
the failure of a quality attribute. Detectability is a mitigating factor in that it
facilitates the identification of potential problems and permits corrective actions to
be performed to preclude the product and/or release of a nonconforming product.
Taken together, the three factors, severity, likelihood, and detectability define
the criticality of a process parameter. In an FMEA analysis, the product of the





















Search WWH ::

Custom Search