Biomedical Engineering Reference
In-Depth Information
9.4 RISK MITIGATION
The goal of any risk assessment is not merely to understand the risks associated
with a particular situation. One is expected to use the assessment to reduce those
risks to the extent possible through suitable means. In truth, much greater empha-
sis should be placed on that aspect of risk management as it provides the desired
benefits of the entire exercise. The use of the aseptic process entails careful con-
sideration of the contributing factors that influence the outcome of the process.
In 1987, the FDA offered this definition/description of aseptic processing:
“In aseptic processing, the drug product, container and closure are subjected
to sterilization processes separately and then brought together. Because there is
no further processing to sterilize the product after it is in its final container, it is
critical to the maintenance of product sterility that containers be filled and closed
in an environment of extremely high quality [34].”
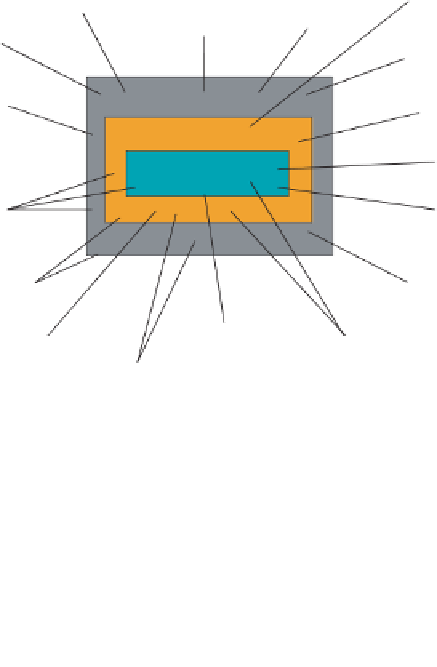
Within this definition are found the essential elements necessary for aseptic
processing: facility, environment, equipment, containers/closures, product proce-
dures, and personnel. This can be visualized in Figure 9.2.
Each aspect is a necessary part of the overall process, and inattention to them
can adversely impact outcome. The contamination potential associated with each
of these elements is certainly variable, but nevertheless attention must be paid to
each to achieve success. The foundation for these controls can be found in the
CGMP regulations, with some of the key elements associated with each outlined
in the following summaries. The following list is not intended to be all inclusive;
Equipment design
Facility
design
Effects from
adjacent
areas
Seasonal
Effects
Personnel traffic
flow
Disinfection
Area
equipment
HVAC
Environment
Equipment
Storage
conditions
Validation
Product
Personnel
practices and
training
Product and
material flow
Personnel
hygiene
Cleaning and
maintenance
Product and materials
Procedures
Sterilization
Qualification
Adapted from Leonard Mestrandrea.
Figure 9.2
Factors influencing aseptic processing. (
See insert for color representation of
the figure
.)























Search WWH ::

Custom Search