Biomedical Engineering Reference
In-Depth Information
Weigh starting
materials
Drying
Add
solvent
Granulation
Mixing
Hold
Tableting
Testing
Coating
Packaging
Testing
Storage and
distribution
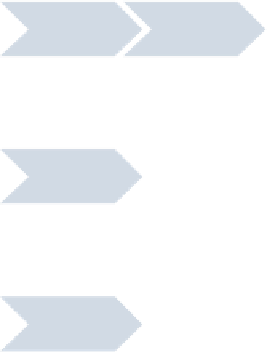
Figure 6.2
Process flow for manufacture of tablets. (
See insert for color representation
of the figure
.)
or fishbone diagram to break down the process into smaller steps and identify
risks associated with each part of the process. The purpose of this assessment is
to prepare a first draft of the product and process control strategy by identifying
processing and product-related risks that are readily apparent and require controls
to be implemented in the manufacturing process. This will ensure that the finished
product will meet its critical and other quality attributes.
Figures 6.2 and 6.3 show, respectively, a block diagram for tablet manufac-
ture and an Ishikawa (fishbone) diagram breaking the process into the 6M's:
Man, Machines, Measurement, Materials, Methods, and Management. These dia-
grams identify risk factors that need to be considered and possibly controlled in
designing the manufacturing and control process.
A multi-disciplinary team, which could include personnel from production
operation, maintenance, Human Resources (HR), R&D, Quality, Analytical and
Microbiological, engineering, purchasing, and marketing, should participate
in the brainstorming session. The more the disciplines represented, the more
effective the session. The idea is to identify as many potential hazards as
possible, working in a systematic manner through the process flow diagram.
It is not appropriate for the Quality Unit to sit in their office and develop
a risk assessment on their own! For example, including a representative
from HR brings objectivity and a completely different skill set to the
brainstorming session. Issues such as process flows and ergonomics from

































Search WWH ::

Custom Search