Biomedical Engineering Reference
In-Depth Information
4.10
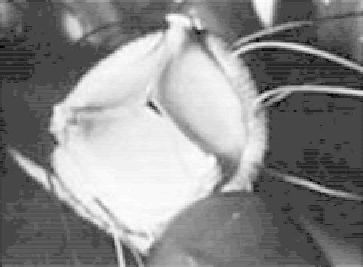
Ionescu-Shiley pericardial bioprosthesis ready for implantation.
4.4.2 Pericardial prosthesis
Pericardial tissue valves (Fig. 4.10) were fi rst designed and manufactured
in Ionescu's laboratory in Leeds, England. Ionescu
et al.
reported an initial
clinical experience from 1971 to 1975, with 142 aortic, 67 mitral, and 3 tri-
cuspid replacements.
61
Embolic rates were low at 0.62% with excellent
hemodynamic function.
62
More recent trials reported that some valves may
develop late disruption of the fi brocollagenous structure, leading to valve
failure.
63
The manufacture of these valves was transferred to California
Shiley Laboratories in 1975. Currently the valves are manufactured by
Edwards Lifesciences.
4.4.3 Carpentier-Edwards porcine bioprosthesis
The Carpentier-Edwards (CE) porcine bioprosthesis (Edwards Life-
sciences, Irvine, CA) has undergone several design refi nements to improve
its hemodynamic performance and increase its durability. The fi rst genera-
tion CE valve was introduced in 1971 and used clinically until 1975. It
consisted of an aortic valve xenograft mounted on a fl exible Elgiloy stent
covered with porous knitted Tefl on. This valve was fi xed with glutaralde-
hyde at high pressure. In 1981 the second generation CE valve was intro-
duced, which had an advanced process for preservation and low pressure
fi xation. The current third generation CE bioprosthesis is fi xed in 0.625%
glutaraldehyde at low hydrostatic pressure and subjected to anti-calcifi ca-
tion treatment. The scalloped shape of the sewing ring facilitates supra- or
intra-annular aortic implantation. By cross-linking the collagen molecules,
the glutaraldehyde stabilizes the integrity of the valve tissue lattice.
64
To evaluate the effect of low pressure fi xation on valve durability,
Fernandez
et al.
retrospectively compared series of CE mitral valves fi xed
Search WWH ::

Custom Search