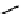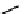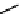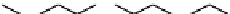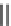Biomedical Engineering Reference
In-Depth Information
O
O
O
H
N
H
N
H
N
H
N
N
H
N
H
N
H
O
O
O
CH
3
CH
3
OH
O
O
O
H
N
H
N
H
N
H
N
N
H
N
H
N
H
O
CH
3
O
CH
3
O
HO
O
O
O
H
N
H
N
H
N
H
N
N
H
N
H
N
H
O
CH
3
O
CH
3
O
OH
FIgurE 18.10
The molecular structure of Silk II. The antiparallel arrangement of β-sheets, indicated by broad
gray arrows, connected via hydrogen bonds (thin dashed lines) makes the material SHG-active.
Silk fibroin can be described by two structural models; Silk I that consists of type II β-turn random
coil domains together with structures including alpha helices and Silk II that is assembled of antiparallel
β-pleated sheets (cf. Figure 18.10) linked with hydrogen bonds giving the material its high mechanical
strength. The SHG signal obtained in silk fibroin assemblies of different structure has been investigated
(Rice et al. 2008) and it was identified that the ordered β-sheet arrangement of Silk II makes this type
SHG-active (cf. Figure 18.10). Results from their investigation are shown in Figure 18.11, presenting
FIgurE 18.11
Characterization of silk fibroin materials of different structures using SHG microscopy. (a) SHG
image measured on an array of silk fibers with high degree of β-sheet arrangement (b) resulting in efficient signal
generation. Whereas structures with a randomized β-sheet orientation such as aqueous films or gels result in no SHG
signal generation, an SHG signal can be detected in compressed and stretched film (c) that has retrieved β-sheet order-
ing (d). (e) SHG image measured on a porous silk scaffold having local regions of β-sheet alignment around the pores
(f), making the pore edges visible in the SHG image. (Adapted from Rice W.L. et al. 2008.
Biomaterials
29:2015-24.
Images kindly provided by Mr. William Rice and Associate Professor Irene Georgakoudi, Tufts University.)