Biomedical Engineering Reference
In-Depth Information
→
→
^
E
ϕ
PMT
α
Excitation
beam
Collagen
Polarizer
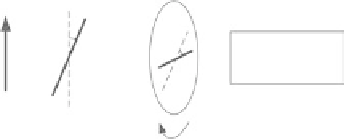
FIgurE A.1
(Reprinted from Han, X. 2011. Novel techniques for quantitative second harmonic generation
microscopy. PhD dissertation, University of Rochester. Ann Arbor: ProQuest/UMI. Copyright 2011 with permis-
sion of the author.)
Z
χ
Z
χ
β
θ
Fibril
Collagen triple-helix
(single helix represented)
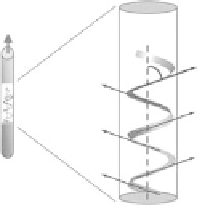
FIgurE A.2
Model 1, θ as helical pitch angle. (Reprinted from Odin, C. et al. 2008. Collagen and myosin char-
acterization by orientation field second harmonic microscopy.
Opt Express
,
16
:16151-16165, Copyright 2008. With
permission of Optical Society of America.)
So, we have
2
(
a
+
c
)cos
2
ϕ
+
b
I
I
y
x
(A.6)
=
2
c
sin(
2
ϕ
)
2
As illustrated in Figure A.2, if the collagen fibril (Figure A.2,
left image
) is considered to be a cylin-
drically symmetric collection of single-axis scatterers (e.g., a collection of helical turns from collagen
single helices, three of which intertwine to form the superhelical collagen triple helix) with a constant
polar angle θ (Figure A.2,
right image
) and a random azimuthal angle φ, there are only two independent
elements of χ
(2)
, the nonlinear susceptibility tensor, with the result that
a
=
n m
−
3
c
=
2
b m
=
(A.7)
n
=
χ
( )
2
=
N
cos
3
θβ
zzz
m
=
χ
( )
2
=
χ
( )
2
=
N
/
2
cos
θ
sin
2
θβ
zxx
xxz
where β is the hyperpolarizability of these individual single-axis scatterers (B,
right image
),
of density
N
. This simplification signifies that the susceptibility tensor has Kleinman symmetry