Biomedical Engineering Reference
In-Depth Information
characterize how the SHG signal builds up at macromolecular scale, that is at the scale of the focal vol-
ume in SHG microscopy.
15.2.1 Molecular origin of collagen nonlinear Response
At the molecular scale, SHG is related to the presence of polarizable electrons in a noncentrosymmet-
ric environment, usually between electron donor and electron acceptor chemical groups (Oudar and
Chemla 1977). Polarization of these electrons by a strong electric field (such as the one in a multiphoton
microscope) results in a nonlinear behavior with components at the second-harmonic frequency since
electronic oscillations are favored toward the electron acceptor group. The question is then to identify
these so-called “harmonophores” in collagen. Aromatic amino acids have been shown to exhibit a sec-
ond-order nonlinear response (Duboisset et al. 2010), but they are almost absent from the amino-acid
sequence of the collagen triple helix that is mainly composed of glycine, proline, and hydroxyproline.
Moreover, SHG signals have been recorded for various types of collagen from many different mammals,
independently of the precise amino-acid sequence.
It was therefore proposed that the second-harmonic response of collagen originates in the peptide
bond itself (Plotnikov et al. 2006, Tiaho et al. 2007, Han et al. 2008, Deniset-Besseau et al. 2010). This
noncentrosymmetrical chemical bond of the peptide backbone indeed exhibits π-electrons delocalized
between C=O and N−H groups that behave as slight electron donors and electron acceptors, respectively
(see Figure 15.1a) (Levine and Bethea 1976). This assertion is supported by polarization-resolved SHG
images of collagenous tissues and polarization-resolved hyper Rayleigh scattering (HRS) measurements
of collagen solutions (Plotnikov et al. 2006, Tiaho et al. 2007, Han et al. 2008, Deniset-Besseau et al.
2010). These experiments measure nonlinear responses for excited fields whose polarization is parallel
or perpendicular to the fibrils main axis. Their ratio can be related to the direction of the nonlinear
electronic oscillation within the harmonophore. They consistently show that this nonlinear electronic
oscillation is oriented along the peptide backbone in collagen.
15.2.2 Building the SHG Signal at Macromolecular Scale
SHG is a
coherent
second-order nonlinear process, which means that it builds up as the summation
of all the second-harmonic electric fields radiated by all the harmonophores within the focal volume.
-
(a)
(b)
O
C
α
+1
+
H
C
α
+1
C
C
N
H
C
α
C
α
(c)
…
…
δ
E
2ω
+δ
E
2ω
+
+ δ
E
2ω
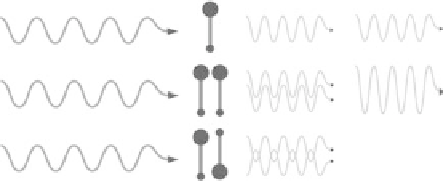
FIgurE 15.1
Physical origin of SHG signal in collagen. (a) Chemical structure of the peptide bond showing
delocalization of π-electrons in a noncentrosymmetric environment. (b) Scheme of second-harmonic response
from 1 isolated harmonophore, 2 harmonophores with the same direction, and 2 harmonophores with antiparallel
directions. In the last case, the 2 harmonic fields have opposite phases and cancel out while the harmonic fields
radiated from 2 parallel harmonophores interfere constructively, which results in a doubled harmonic field. SHG
intensity that is obtained as the square of the total harmonic field exhibits a quadratic dependence as a function
of the number of aligned harmonophores. (c) Scheme of coherent amplification of harmonic response along the
rigid and compact collagen triple helix. (Adapted from Strupler, M. et al., 2008.
J. Biomed. Optics
13:054041. With
permission of SPIE.)