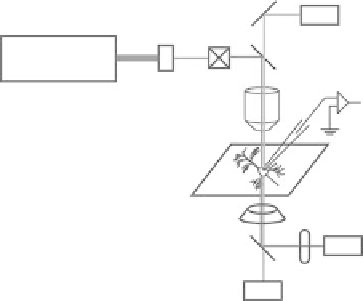
Biomedical Engineering Reference
In-Depth Information
(a)
TPF signal
(b)
DM
PMT
Galvano
mirrors
AOM
Femtosecond
Laser (1000 nm)
DM
Amplifier
(Electrophysiology)
Stimulation group
Control group
Objective
lens
×50 cycles
Recording
chamber
10 msec
15 msec
Stimulation
30 msec
Condenser
Laser illumination
SHG signal
PMT
DM
500 msec
500/10 nm
Bandpass filter
Interval between laser pulses
TPF signal
PMT
FIgurE 10.1
Schematic diagram of the experimental setup for imaging membrane potential with SHG.
(a) Schematic diagram of the SHG setup. Neurons are placed in a recording chamber under the regular two-photon
microscopy setup equipped with femtosecond laser and SHG detector in the transmission path. Electrophysiological
and optical signals are recorded simultaneously. AOM, acousto-optic modulator; DM, dichroic mirror; PMT, pho-
tomultiplier tube. (b) Schematic diagram of the point-scan protocol for measuring fast electrical events. In the
point-scan protocol, the laser position is fixed at a single point and the laser is illuminated on the sample for a
short period of time with an interval between illuminations. In this diagram, laser is illuminated on the sample
for 30 ms with 500 ms of interval in between illuminations. In the typical experiments, neurons are stimulated
electrophysiologically during the laser illumination and the SHG signals during this period are compared to those
without stimulation.
photons of exactly half the wavelength of illuminating laser to be detected in the SHG channel while
longer wavelength TPF photons are detected in the other detector. The numerical aperture of the con-
denser should at least match that of the objective lens to collect all the SHG photons generated from the
cone-shaped incoming photons [19]. Even higher NA does not dramatically help in theory as well as in
practice under our experimental conditions, but one can expect that it may increase photon collection
in detecting SHG signals from highly scattering samples.
10.4.2 imaging electrical Signals with SHG
SHG images can be obtained in any mode, but to image membrane potential dynamics, there are some
factors that need to be considered. Although it varies on the preparation and temperature, action poten-
tials are very fast phenomena, where the entire events can be finished in the order of milliseconds. As
such, the detection and recordings of SHG signals need to be faster than a millisecond to capture these
events. This is challenging for the regular two-photon imaging regime (frame-scan) as laser scanning
is a relatively slow process. This can be overcome by acquiring whole areas of images and focusing the
recording sites to a single line (line-scan) or even a single point (point-scan). With the advancement of
devices, the line-scan can now be performed at a rate faster than 1 kHz (i.e., submillisecond). However,
faster scanning means less pixel dwell time and therefore less signal photons from a single point at a
single time, which, in the end, reduces the signal-to-noise ratio. Therefore, continuous recording from
single point with laser illumination at a fixed point has a big advantage in this regard. In this case, the
temporal resolution is limited only by the recording device itself. In fact, we normally use point-scan
to detect fast SHG changes induced by fast electrical events such as action potential. However, longer
pixel dwell time means more photon-induced damages at the site of laser focus. This is especially true
for two-photon imaging as it utilizes high power lasers to achieve rare two-photon events. In theory, if
laser illumination only produces SHG signals without absorption of photon energy by chromophores
(i.e., fluorescence), it should not induce any photodamage. However, in reality, because of resonant