Biomedical Engineering Reference
In-Depth Information
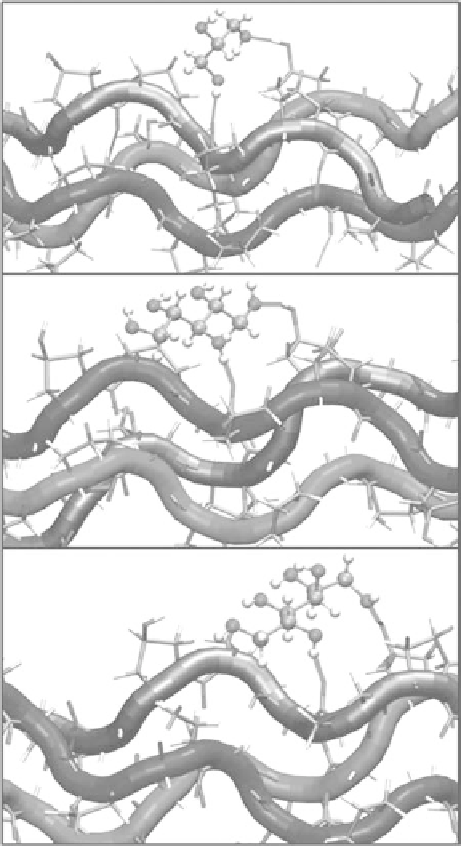
simulation results, the position of hydroxyl (OH) groups on clearing agent molecules imposes steric
constraints on surface bridge formation on collagen triple helix (see Figure 8.6). This finding was vali-
dated by experimentally observing the kinetics of skin dehydration by 1,2- and 1,3-propanediol. These
two agents have very close molecular weights, refractive indices, and osmolarity, but the 1,3-isomer was
found to be more effective after the first hour of clearing. Meanwhile, the steady state after 24 h of clear-
ing showed no difference between the two agents. Additionally, formalin fixation was shown to reduce
the efficiency of optical clearing by glycerol in rodent skin and collagen gel [38]. However, since clearing
is ultimately achieved in fixed tissues (albeit at much longer exposure times), Hirshburg et al. [37] sug-
gested that the destabilization happens at the molecular level and not at the fiber scale.
(a)
(b)
(c)
FIgurE 8.6
Typical hydrogen bond bridges in alcohols. The bridge of −OH groups between (a) one and three
carbon positions (type II) in glycerol, (b) one and three carbon positions (type II) in xylitol, and (c) one and five car-
bon positions (type IV) in sorbitol. Higher bridge types, as in (c), span further across the collagen surface and can
potentially disrupt collagen-collagen and collagen-water interactions better than lower bridge types (Hirshburg,
2010). (Reproduced with permission from Hirshburg, J. M. et al. 2010.
J. Biomed. Opt.
15.)