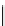Biomedical Engineering Reference
In-Depth Information
Flow chart
Start
Launch photon packet through
objective lens to focal plane
z
f
Determine transmittance
T
ω
(
z
f
)
Determine fraction of
T
ω
(
z
f
)
within SHG cross section
σ
T
ω
(
z
f
,
σ
)
Set
W
p
(2
ω
) =
T
2
(
z
f
,
σ
)
ω
Set initial SHG emission direction
F
SHG
=
X
,
B
SHG
= 1 -
X
Determine fraction of packet
exiting tissue,
T
(2
ω
),
R
(2
ω
)
Determine SHG components
forward/backward ratio
F
/
B
=
T
(2
ω
)/
R
(2
ω
)
forward attenuation at
z
f
T
(2
ω
,
z
f
)
Y
Another
z
f
N
End
FIgurE 8.2
Flowchart of the algorithm used in the Monte Carlo simulations of the axial dependences of the
measured SHG directionality and attenuation. (Reprinted from
Biophys. J
., 94, Lacomb, R., O. Nadiarnykh, and P. J.
Campagnola. Quantitative SHG imaging of the diseased state
Osteogenesis imperfecta
: Experiment and simulation,
4104-4104, Copyright 2008, with permission from Elsevier.)
by the bulk optical properties at the SHG wavelength. Analogously, these simulations can be done for
bulk spectral measurements [31]. In the following discussion of optical clearing in muscle and tendon
simulation, the results will be addressed along with the experimental data.
8.4 Mechanism of optical clearing in Muscle
Optical clearing with glycerol significantly improves the imaging depth in striated muscle as shown
in Figure 8.3, where the
xz
projections are reconstructed from optical slices imaged with SHG, and
muscle cells are visible as deep as 500 μm [6]. In this work,
ex vivo
muscle slices were treated for several
hours with 25%, 50%, and 75% glycerol solutions resulting in the axial attenuation profiles shown in
Figure 8.3b in comparison with untreated (control) muscle. As a side effect, glycerol penetration results