Biomedical Engineering Reference
In-Depth Information
It is also illustrative to relate the normalized domain used in Figure 6.2b to λ
SHG
with experimental
observations that fibrils on the order of λ
SHG
/10 produce creation ratios of
F
SHG
/B
SHG
~ 1. We point out
that based on Equation 6.8, the forward emission coherence length is affected by both
K
and Δ
k
(which
is at least Δ
k
1
). To justify a forward coherence length on this order, we note that the maximum coher-
ence length as limited by dispersion is on the order of 7 μm and any axial momentum contribution from
the medium acts to decrease this value, potentially by an order of magnitude. Thus, by normalizing the
domain to the SHG coherence length, we observe that for domains on the order of λ
SHG
/10 (normal-
ized to 0.1 in Figure 6.2b), the %
F
SHG
is close to 50%, as suggested by other work [2,6] and increases to
approximately 100%
F
SHG
for domains on the order of λ
SHG
(normalized to 1 in Figure 6.2b).
6.3.4 Fiber Morphology
It has been suggested in other work that differences in forward and backward detected morphology are
most likely to be manifested in the observation of smaller features. Specifically, several reports [2,24]
have shown the existence of segmented appearing fibrils in the backward channel, where these same fea-
tures appear to be continuous in the forward geometry. We can now explain this phenomenon in terms
of the difference in forward and backward coherence lengths associated with the respective relaxed
phasematching considerations and intensity amplification due to QPM. As an example, we show the
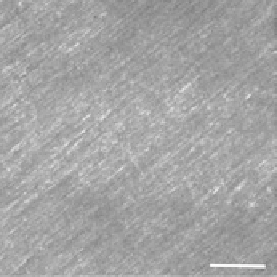
forward and backward collected images from
Valonia
cellulose in the left and right panels of Figure 6.4.
These specimens are approximately 30 microns in thickness, and the MFP is ~130 microns [24]. Thus,
the contrast in the backward channel arises predominantly from direct quasicoherent emission and
will not contain a significant multiply scattered contribution. We note that the fibrils observed in the
forward channel are long and continuous, whereas these frequently have a segmented appearance in
the backward channel. This can now be interpreted by our assignment of
F
SHG
and
B
SHG
with relatively
small and large Δ
k
values, respectively, which assigns a shorter coherence length to the latter. This result
predicts that if the fibril packing in the axial direction is on the order of the backward coherence length,
destructive interference occurs for the backward signal. By contrast,
F
SHG
is characterized by a relatively
longer coherence length, and such destructive interference between fibrils does not contribute to the
forward contrast. In other word, the fibrils are not physically segmented but their appearance as such in
the backward channel arises from the quasicoherence of SHG in tissue.
This can also explain the findings of Williams et al. [2], where they showed that the morphology in
mature rat tail tendon fibrils displayed similar features in the forward and backward collection geom-
etries, whereas immature fibrils had a similar, segmented appearance. We suggest this arose because
the immature tendons have effectively smaller domains and interfibril spacing. We further suggest the
(a)
(b)
FIgurE 6.4
Forward (a) and backward (b) SHG images of
Valonia
cellulose. Segmented features appear in the
backward channel due to destructive interference. Scale bar = 20 microns. (From Lacomb, R. et al. 2008.
Opt.
Commun
. 281:1823-1832. With permission.)