Biomedical Engineering Reference
In-Depth Information
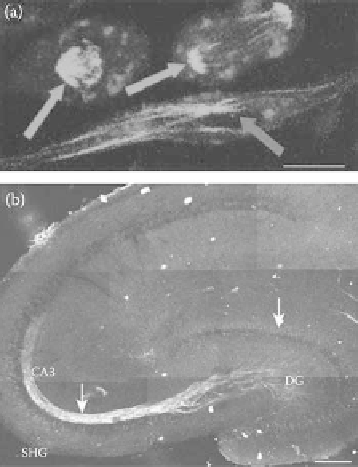
FIgurE 5.12
(
See color insert.
) SHG from microtubules. (a) SHG in RBL cells. SHG (green) arises from mitotic
spindles (orange arrows) and from interphase microtubule ensembles (blue arrow). Scale bar: 10 μm. (b) SHG in
hippocampal brain acute slice. SHG arises from the dense mossy fiber axon bundle between the dentate gyrus (DG)
and CA3 rea of the hippocampus. Scale bar: 200 μm. (Modified from Dombeck, D.A. et al. 2003.
Proc Natl Acad
Sci 100
, 7081-7086.)
Molecules with π-electron donors and acceptors exhibiting intramolecular charge transfer between
the two groups show large nonlinear second-order optical susceptibility (Lalama and Garito, 1979).
The amide HRS is due to the partial charge transfer in the peptide bond due to two resonance forms,
as shown in Figure 5.13. As a consequence of this resonance, all peptide bonds are found to be almost
planar.
SHG has been experimentally observed in polypeptide α-helix (Mitchell et al., 2005). An α-helix is a
tightly coiled structure, which has an average of 3.6 amino acids per turn, pitch of 5.5 Å, and radius of
2.2 Å. An individual α-helix, therefore, is characterized by cylindrical symmetry with all HRS emitters
tilted at a fixed polar angle with respect to the helical axis (the same geometry described in Section 5.3).
The generation of SHG signal through coherent summation requires an anisotropic distribution of the
scatterers. Proteins characterized by randomly oriented α-helices do not fulfill such anisotropy and are
not expected to be good SHG emitters. On the other hand, proteins with a high degree of alignment of
their α-helices should produce coherent summation. In other words, a first level of order (required for
constructive interference) is achieved by organization of peptide bonds in a helical pattern; however, a
second level of order is also necessary, consisting of substantial alignment of the helices themselves in
the protein.
O
-
O
R
R
N
N
C
C
H
H
R
′
R
′
FIgurE 5.13
Resonance structure of the peptide bond. Delocalization of π-electrons allows charge transfer
between the donor N and acceptor O.