Biomedical Engineering Reference
In-Depth Information
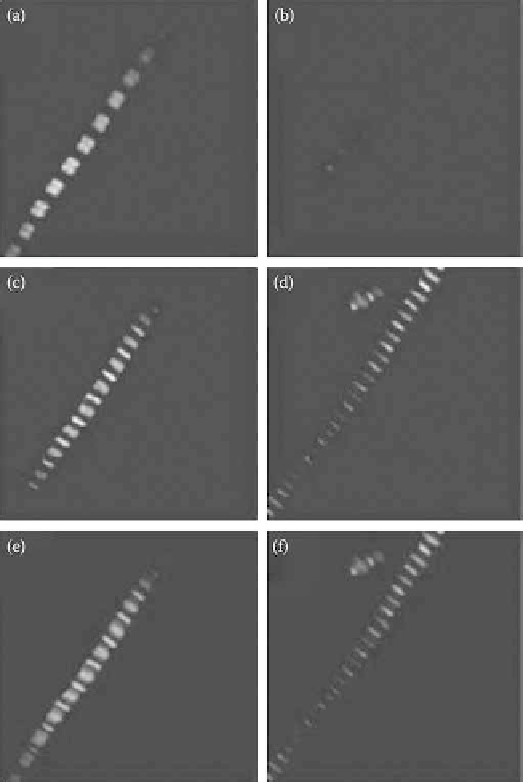
FIgurE 5.9 (See color insert.)
Effect of myosin extraction on SHG from myofibrils. Panels a and b show myo-
fibril SHG images before and after myosin extraction, respectively. Panels c and d show two-photon excitation
images of actin labeled with rhodamine-phalloidin before and after myosin extraction, respectively. Panels e and f
show a superposition of SHG image (in red) and TPE image (in green) before and after the extraction. The size of the
images is 20 × 20 μm
2
. (Modified from Vanzi, F. et al
.
2006.
J Muscle Res Cell Motil 27
, 469-479. With permission.)
that the structural changes occurring in myosin S1 during force production can be monitored by SHG.
During contraction, the fraction of myosin molecules which interact with actin to generate force can
be modulated by changing sarcomere length and, thus, the degree of overlap between actin and myosin
filaments. Figure 5.10c shows the dependence of γ on sarcomere length at rest (light gray circles) and at
the plateau of isometric contraction (dark gray circles). In the sarcomere length range between 2.2 and
3.6 μm, the fraction of cross-bridges that can attach to actin decreases linearly, as indicated also by the
tetanic isometric force (black squares and lines in Figure 5.10c). The γ
act
data track remarkably the force
behavior, confirming that SPA is sensitive to the fraction of attached myosin heads. As such fraction
goes to zero at no overlap (sarcomere length > 3.6 μm), γ
act
approaches γ
rest
.
Force generation during active contraction occurs in the acto-myosin cross-bridges, which sample
different biochemical/structural states during the myosin ATPase cycle. By depleting ATP from the
muscle fiber, it is possible to induce a static and structurally homogenous state (the rigor state) in which