Biomedical Engineering Reference
In-Depth Information
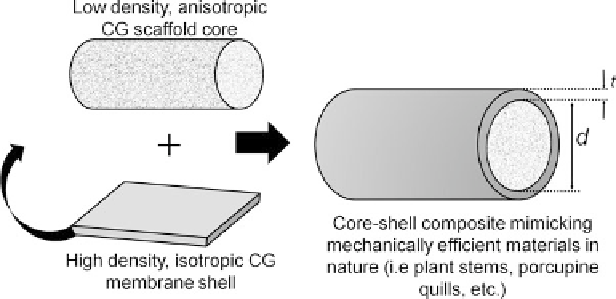
Fig. 16.5
Schematic of CG core-shell composite design
pulposus integrated with layered, aligned nanofiber mats mimicking the annulus
fibrosus region [
74
,
75
].
The CG scaffold-membrane composite paradigm was developed to avoid the
typical tradeoff for porous tissue engineering scaffolds between mechanical
properties (Young's modulus:
E
*) and bioactivity (permeability:
k
and porosity:
e
).
Briefly, previous experimental and cellular solids modeling studies have shown
that scaffold modulus increases with (
r
*/
r
s
)
2
(and is not affected by pore size)
but that scaffold permeability increases as (1
(
r
*/
r
s
))
3/2
[
57
,
58
]. Hence, to
increase CG scaffold elastic modulus by the two or three orders of magnitude
necessary for tendon applications (minimum 100 MPa hydrated modulus), the
corresponding increase in relative density would have adverse effects on overall
scaffold bioactivity (permeability and porosity). Taking inspiration from core-
shell structures in nature [
56
], the scaffold-membrane composite design can help
overcome these limitations.
16.4.1 Fabrication of Aligned CG Scaffold-Membrane
Composites via Liquid-Solid Phase Co-synthesis
The core-shell CG composite paradigm was implemented using the same
technologies developed to create multi-compartment CG scaffolds for
osteochondral tissue engineering. Here a high density (high tensile strength) CG
membrane was combined with a low density (porous) aligned CG scaffolds into a
single biomaterial via a liquid-solid phase co-synthesis method [
1
,
47
] (Fig.
16.5
).
As a precursor for using these composites for tendon repair, the scaffold core
contained a longitudinally aligned, anisotropic pore microstructure fabricated
using a recently developed directional freeze-drying approach [
53
]. The aligned
microstructure was hypothesized to improve construct regenerative capacity by
increasing the modulus in the direction of alignment and by providing contact
Search WWH ::

Custom Search