Biomedical Engineering Reference
In-Depth Information
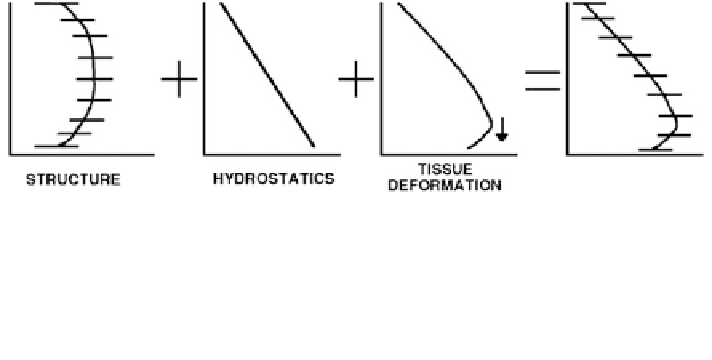
Fig. 5 The multiple contributing factors to the distribution of pulmonary blood flow. Vascular
structure results in a heterogeneity in the distribution of blood flow, and long path lengths far
from the heart result in a reduction of blood flow in extremities. The hydrostatic pressure gradient
acts to induce a gravitational gradient in blood flow by acting directly on blood to drive it
preferentially to the base of the lung. Finally, lung tissue deforms downwards, which increases
the flow gradient and contributes to the size of zone 4. This figure was first published in
Pulmonary Circulation [
83
]
The ladder-like representation of the intermediate and micro-vessels [
31
] has
enabled bridging of the gap between arteries and veins in large-scale anatomically-
based mathematical models of the pulmonary circulation [
17
], hence negating the
constraint to prescribe boundary conditions at the pre-capillary level in both
macro- and micro-scale models. This new model, which spanned the important
spatial scales in the lung, is illustrated in Fig.
4
. It was able—for the first time—to
assess the relative contributions of the compound effects of gravity on pulmonary
blood flow gradients and heterogeneity. In particular the model addressed the
importance of (1) structure, (2) the hydrostatic pressure gradient in the lung, and
(3) tissue deformation to blood flow distribution. Figure
5
provides a schematic of
how each factor contributes to the overall distribution of perfusion. Analysis of the
full model confirms that heterogeneity is introduced into the system via
the asymmetric structure of the pulmonary blood vessels, as each path to and from
the capillary beds has a unique resistance to flow. This means that even in zero
gravity (0G) there is significant heterogeneity in the distribution of perfusion. The
model predicts that heterogeneity is *47 % of its 1G value in 0G conditions. The
hydrostatic pressure gradient acting directly on blood establishes a gravitational
gradient in the distribution of perfusion, and introduces a height-dependent pres-
sure gradient at the capillary level. Even without tissue deformation, this may be
responsible for up to 80 % of the overall gravitational gradient in perfusion. This
gradient contributes to overall heterogeneity, in the sense that variation of per-
fusion in the lung as a whole increases with the introduction of gravitationally
varying pressures. However, local heterogeneity is a function of blood vessel
branching asymmetry. Finally, the effect of gravitational deformation of lung
tissue acts to alter this gravitational gradient, normally increasing its magnitude.
Tissue deformation also contributes (along with structure) to an observed decrease
in blood flow rates in gravitationally dependent tissue. This phenomenon is known
as ''zone 4'' [
81
], and is only predicted in theoretical models that include an
asymmetric geometry. The model suggested that upstream resistance, which is
Search WWH ::

Custom Search