Biomedical Engineering Reference
In-Depth Information
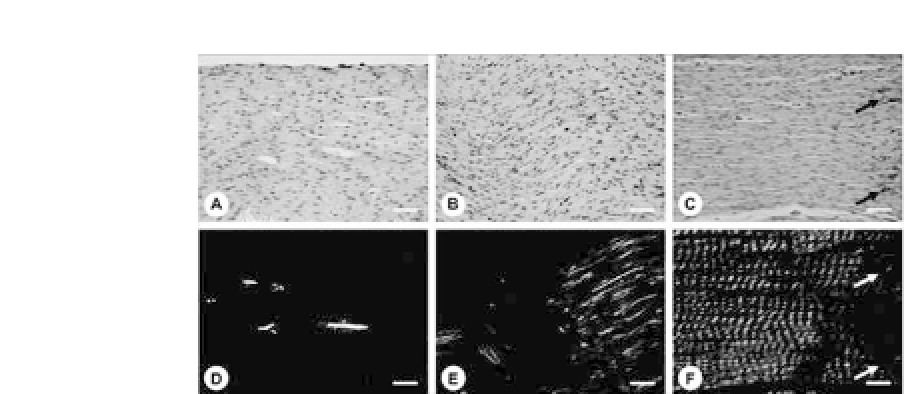
Figure 34.5.
H&Estaining(a-c)andpolarizedmicroscopicviews(d-f)of
in vitro
-engineered tendon after total 12 weeks (a and d) and of
in vivo
-
implanted tendons for 14 weeks without (b and e) and with (c and f) load-
ing.Originalmagnifications:
200;barrepresents50
μ
mforall.(Reprinted
by permission from Ref. 13). See also ColorInsert.
×
collagen fibers, a more mature collagen fibril structure with D-band
periodicity, and stronger mechanical properties (Fig. 34.5).
These results indicate that
in vivo
mechanical loading via an
ex
vivo
approach might be an optimal approach for engineering func-
tionaltendontissue.Therefore,areasonablestrategyforengineered
functional repair of tendon defects might be to generate a neo-
tendon tissue first
in vitro
and then to implant
in vivo
for its further
maturation and for carrying out itsfunctions.
Thephysicalformofpolymerfibersmayalsoaffecttheirmechan-
icalpropertyandlikelytheirdegradationrate.Apreviousstudyper-
formed in our center found that once in a woven fashion, PGA fibers
could significantly enhance their mechanical strength. Additionally,
the woven fibers also seemed to degrade more slowly compared
withnonwovenfibersat the same time points.
14
34.3 PGA Scaffold for Cartilage Engineering
Cartilage engineering is another major area of tissue construc-
tion. Engineering of hyaline cartilage to repair large, full-thickness
defects of articular cartilage became the first target because the
defect remains to be a major concern in clinical practice due to the
lack of proper therapy.
15
In a porcine model, autologous articular







Search WWH ::

Custom Search