Biomedical Engineering Reference
In-Depth Information
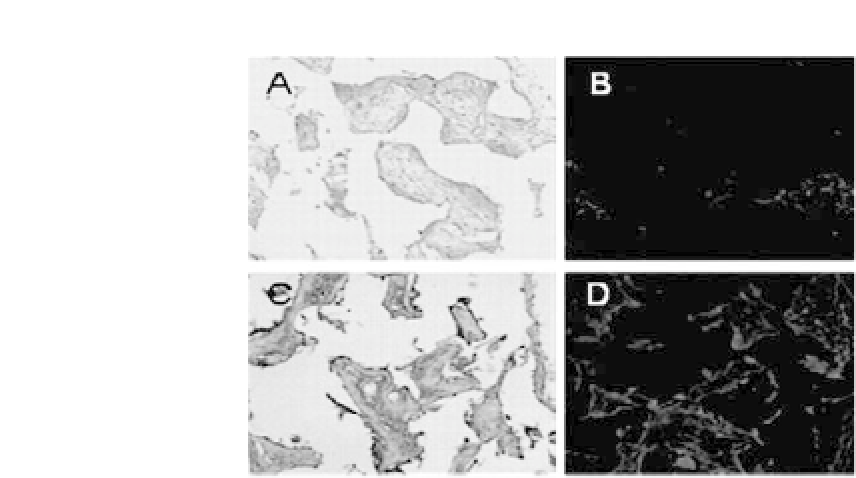
Figure 27.5.
In vivo
evaluation of SMC-seeded PLCL scaffolds. CM-DiI-
tagged SMC-seeded PLCL scaffolds were subcutaneously implanted in nude
mice for 2 (a, c) and 8 weeks (c, d). (a, c) Immunohistochemical staining for
SM a-actin and (b, d)CM-DiIdetection.
induce the phenotype of SMCs
in vitro
to be similar to that of SMCs
in vivo
. Aortic SMCs were seeded onto PLCL scaffolds and sub-
jectedtopulsatilestrainincultureinpulsatileperfusionbioreactors.
SMCs proliferate and eventually cover the surface of the scaffolds
over eight weeks in pulsatile perfusion bioreactors. In control, sta-
tic culture, cells grow much slower and the scaffold surface was not
completely covered at eight weeks. Pulsatile strain enhances SMC
proliferation and collagen production. In additional to collagen,
elastin is a major ECM component secreted by functional SMCs
in native blood vessels. The elastin content of transplanted PLCL
scaffolds was examined as a measure of SMC differentiation in a
mechanically active culture system. Elastin expression is greater in
the scaffolds exposed to mechanical stimuli relative to that in static
constructs.
31
Western blot analysis demonstrates that the expres-
sion of SM
α
-actin is upregulated by 2.5-fold in SM tissues recon-
structed under the mechano-active conditions compared with that
in tissues grown in static conditions.
18
The study demonstrates
that tissue engineering of SM tissues
in vitro
using pulsatile perfu-
sion bioreactors and elastic PLCL scaffolds leads to enhanced tissue
development and differentiated SMC phenotype.







Search WWH ::

Custom Search