Biomedical Engineering Reference
In-Depth Information
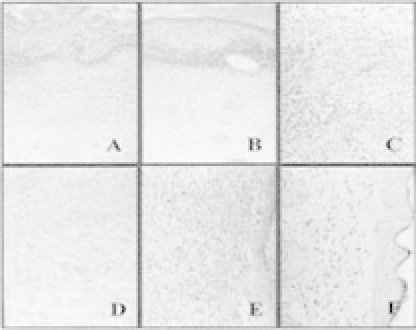
Figure 12.5.
Histological findings of a wound (a) at 3rd day postwound-
ing of the control group (HE stain 200); (b) at 3rd day postwounding,
polyurethane-membranegroup(HEstain200);(c,d)at6thdaypostwound-
ingofthepolyurethane-membrane-treatedgroup(HEstain200);(e)at15th
daypostwoundingofthecontrolgroup(HEstain200);(f)at15thdaypost-
wounding of the polyurethane-membrane-treated group (HE stain 200).
Reprinted from Ref. 14 with permission from John Wiley &Sons Inc.
6 mm in diameter or in areas of low blood flow, mainly due to the
early formation of thrombosis. Moreover, commonly used materials
lack growth potential, and long-term results have revealed several
material-related failures, such as stenosis, thromboembolization,
calcium deposition, and infection. Tissue engineering has become
a promising approach for generating a biocompatible vessel graft
with growth potential. Since the first success of constructing blood
vessels with collagen and cultured vascular cells, there has been
considerable progress in the area of vessel engineering. To date,
tissue-engineered blood vessels (TEBVs) could be successfully con-
structed
in vitro
and be used to repair vascular defects in animal
models. From the days of research on developing vascular grafts
using materials that produce minimal interaction with the inflow-
ing blood and adjacent tissues, researchers have come a long way in
developing constructs at the nano scale that interact with cells and
cause bloodvessel formation.
Conventional electrospinning produces randomly oriented
nanofibers; however, Mo
et al.
developed an aligned biodegrad-
able PLLA-CL (75:25) nanofibrous scaffold using a rotating collec-
tor disc for collection of aligned electrospun nanofibers.
19
These








Search WWH ::

Custom Search