Biomedical Engineering Reference
In-Depth Information
and hydrogel fabrication. The following sessions discuss detailed
chemistries of HA derivatives and their applications in hydrogel
fabrication.
9.3 HA Derivatives
9.3.1
Ester Derivatives
As mentioned in the previous session, HA is a very flexible, vis-
cous,biodegradable,andhydrophilicnaturalpolymer.Toformstruc-
tural rigidity and to be less susceptible to enzymatic degradation,
conversion of hydrophilic HA networks to more hydrophobic ones
wasperformedbychemicalgraftingofhydrophobichydrocarbonsto
the carboxylic acid in HA. The chemical modification was processed
through an alkylation of HA via an alkyl halide, obtaining HA deriv-
atives with different degrees of modifications of its available car-
boxyl groups. Depending on the degree and types of alkylations,
corresponding degrees of rigidity and hydrophobicity of HA were
achieved. As an example, Fidia Advanced Biopolymers (Italy) has
yielded diverse commercial HA esters with hydrophobic domains
(Hyaff-11 by grafting ethyl esters [Fig. 9.2] and Hyaff-11 by grafting
benzyl esters), thus fabricating fiber, membrane, and sponge forms
of HA. The Hyaff-11 microparticles demonstrated better results of
bioactivemoleculesandcelldeliveryandatthesametimehavebeen
employed as scaffolds for cartilage regeneration and mesenchymal
stem cell cultures.
9.3.2
Carbodiimide (R
1
N
=
C
=
NR
2
)
Chemical reactions of HA via 1-ethyl-3-(3'-dimethylaminopropyl)
carbodiimidehave been performed between its carboxylic acid and
amine groups of the targeting bioactive macromolecules. Since the
Figure 9.2.
Example of formation of hydrophobicHA.
16
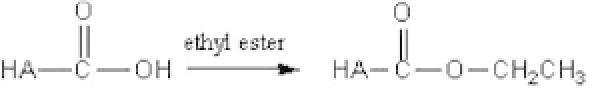








Search WWH ::

Custom Search