Biomedical Engineering Reference
In-Depth Information
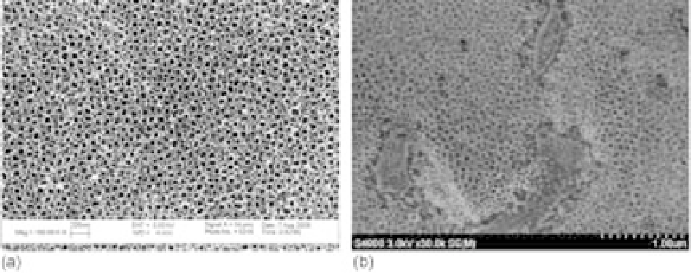
2.2 Scanning electron microscopy (SEM) images of (a) anodized pure
titanium, and (b) anodizedTi-6Al-4V in a dilute fluorine-containing electrolyte.
used and the applied voltage must be much lower than the dielectric breakdown.
For such titanium nanotubular surfaces, apatite layers can be easily formed by
simply soaking anodized crystalline titania in simulated body fluid (SBF)
because anodized titanium with anatase and rutile titania surfaces were shown to
induce apatite formation in vitro (Yang et al., 2004; Oh et al., 2005). This
technique could be useful as well as adherent bioactive nano-HA layers on
titanium-based implants are created which simulate the size and shape of natural
HA in bone.
A preliminary study showed that anodized Ti-6Al-4V in fluorine-containing
electrolyte solutions displayed nanotubular structures similar to the anodized
titanium all over the surface except where the phase was present (Fig. 2.2b).
Both bone and cartilage cells exhibited increased activity on anodized Ti
surfaces compared with untreated Ti (Fig. 2.3). Numerous studies confirmed that
such anodized titanium alloys can promote osseointegration compared with
untreated surfaces (Yao et al., 2008; Das et al., 2009; Lee et al., 2009).
CoCr alloys can also be anodized to possess nanoscale features. A Co-
33.4Cr-3.3Mo alloy was successfully anodized in both fluorine-containing and
fluorine-free acidic electrolytes. The resulting surface possessed numerous
micron features as well as nanoporous structures all over the surface (Fig. 2.4).
The size of such heterogeneous porous structures was about 20 to 30 nm under
10 V. Under lower voltages (2 V), the resulting topography was less rough in the
micron-scale while nano rough features looked similar to the surfaces created
under 10 V (Fig. 2.4). Anodized CoCrMo had an oxide layer composed of CoO,
Co
3
O
4
, and Cr
2
O
3
(Gallant et al., 2006; Surviliene et al., 2008). The network of
±Cr±O±Cr± bridges in the oxide of CoCr alloys are considered to be the main
reason for their passivity (McCafferty, 2002). In addition, due to the formation
of this oxide layer, the water contact angle of anodized CoCrMo was nearly 30ë
lower than the unanodized CoCrMo, and, thus, indicated better wettability of the
CoCr surfaces for protein adsorption and subsequent cell attachment. This is
promising in terms of improving the biocompatibility of CoCr alloys. Pre-