Biomedical Engineering Reference
In-Depth Information
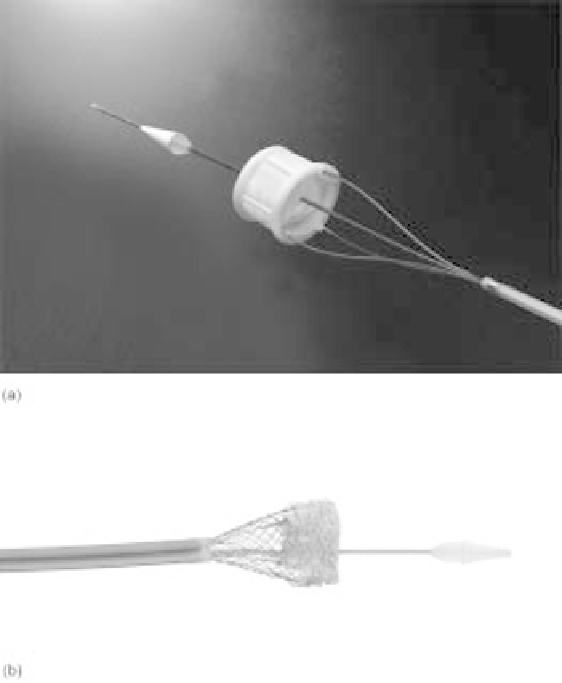
5.21 Two second-generation percutaneous valves in clinical development. (a)
The Direct Flow Medical percutaneous valve, which utilizes an inflatable
polymeric stent and bovine pericardial leaflets. (b) The Sadra Lotus
TM
Valve
System, with a repositionable nitinol stent and bovine pericardial tissue leaflets.
Both valves were designed to enable clinicians to examine initial valve
placement, particularly in relation to the coronary arteries, and reposition, if
necessary, prior to permanent implantation. (Reprinted with permission from
Direct FlowMedical, Inc. All Rights Reserved. Lotus
TM
is a trademark of Sadra
Medical. Reprinted with permission from Sadra Medical. All Rights Reserved.)
Therefore children cannot benefit from traditional bioprosthetic valves and must
use mechanical valves and be subjected to Coumadin therapy, with all the
limitations on their quality of life that the blood thinner imposes.
A key hurdle in advancing tissue engineered heart valves to commercial
reality will be to reduce the technical risks on such a project. An excellent
overview of the challenges for heart valve tissue engineering was recently
published (Sacks et al., 2009b). This review envisions an implantable synthetic
device containing living cells and a supporting biomatrix. Another interesting