Biomedical Engineering Reference
In-Depth Information
5.6.3
Tissue treatments
Once calcification was realized as a primary limitation to long-term valve
durability, the field has focused on various treatments to reduce calcification.
Since those initial studies conducted in the early 1980s, several technologies
have been commercialized, focused on removing phospholipids (Nashef and
Ahmed, 1989; Lentz and Pollock, 1982). These technologies remove the binding
sites for the calcium, and the correlation between quantitative phospholipid
levels and calcification levels has been reported (Cunanan et al., 2001). While
the use of an anionic surfactant has been associated with increased incidence of
thromboembolism clinically, the use of a non-ionic surfactant has been widely
used with the Edwards Lifesciences brand of porcine and pericardial valves
(Hartz et al., 1986). Alcohol also reportedly reduces phospholipid levels in
tissues (Levy and Hirsch, 1998) and has been incorporated in the St. Jude
Medical treatment called Linx
TM
(Connolly et al., 2004).
Other theories have been proposed to explain the pathophysiology of
calcification, as summarized by Schoen and Levy (1999). One such theory that
has reached commercialization is to block residual aldehyde groups using 2-
aminooleic acid (AOA
Õ
) (Girardot, 1990). This technology has been applied to
the Medtronic Mosaic
Õ
and Freestyle
Õ
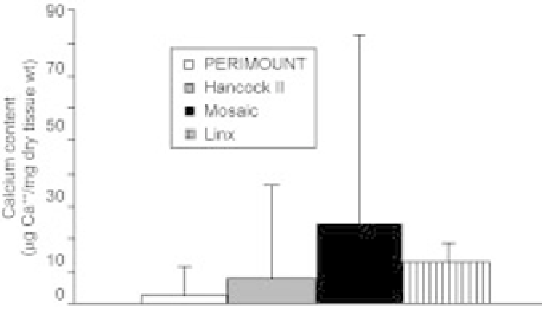
porcine valves (Gott et al., 1992). Figure
5.19 shows the comparative performance of these commercial processes in the
rat subcutaneous model.
Surprisingly, while all treatments were developed with the intent of reducing
calcification, none of the treatments commercialized has been optimized to
++
5.19 Comparative analysis of calciumcontent (mg Ca
/mg dry tissue weight)
for various commercial tissue processes, as evaluated in the rat subcutaneous
implant model. Data for PERIMOUNT
Õ
, Hancock II
Õ
Õ
, and Mosaic
taken from
TM
Cunanan et al. (2001) after 90 days implantation. Data for Linx
taken from
Connolly et al. (2004) after 21 days implantation. (PERIMOUNT
Õ
is a
Õ
registered trademark of Edwards Lifesciences Corporation. Hancock II
and
Mosaic
Õ
are registered trademarks of Medtronic, Inc. Linx
TM
is a trademark of
St. Jude Medical, Inc.)