Biology Reference
In-Depth Information
A
B
Cation-independent
mannose-6-phosphate
receptor
Activated
GPCR
Plasma membrane
Endosomal membrane
VPS29
Sorting nexins
Ub
Adaptin
AP-2
P P
PRLYL
Ub
VPS26
VPS35-N
VPS35-C
β
-Arrestin
P
Clathrin
Golgi proteins
C
D
Jen1
Endosomal vesicle
Ric1
VPS51
VPS53 VPS54
GARP
tethering
complex
VPS52
P
Ub
Rab6
Ub
RGP1
Rod1
P
TGN membrane
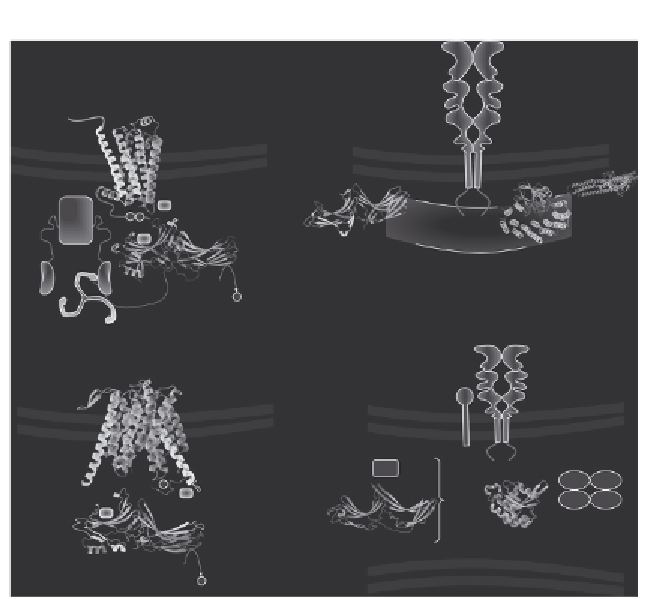
Figure 2.7 Functional context of arrestin-fold proteins. (A) b-Arrestins interact with acti-
vated, phosphorylated GPCRs to interfere with G-protein signaling and to recruit the
endocytic machinery (adaptor protein AP-2, clathrin) to binding sites located on the
C-terminal tail which is unfolded as a result of the receptor binding. Ubiquitination sites
on the GPCR and the b-arrestin are only indicative. (B) VPS26 is a subunit of the pent-
americ retromer (consisting of, besides VPS26, the cargo-loading subunit VPS35, VPS29,
and a pair of sorting nexins harboring PX- and BAR domains) involved in the retrograde
transport of the mannose-6-phosphate receptor from the endosome to the TGN. The
cation-independent mannose-6-phosphate receptor is outlined after a published tenta-
tive view.
154
(C) Rod1-ART4 is dephosphorylated and ubiquitinated by the ubiquitin
ligase Nedd4. It mediates the ubiquitination and the surface removal of the MFS family
protein Jen1. Rod 1 and Jen1 were modeled with Phyre2. It is uncertain where Rod1 acts.
Phosphorylation and ubiquitination sites on Jen1 and Rod4 are only indicative. (D) RGP1
forms together with Ric1 a heterodimeric GEF for Rab6. They regulate the tethering
complex GARP-dependent fusion in a Golgi retrograde transport mechanism.
proteins have important extensions outside the arrestin core, organized or
not as known domains. The structure of the arrestin core, although modeled
with a high confidence level (
95%) represents only part of the whole struc-
ture and the function of these arrestin-related proteins undoubtedly relies on
the integration of the various structural portions.
Search WWH ::

Custom Search