Biology Reference
In-Depth Information
LIM
kinase
Slingshot
P
P
Cofilin
Cofilin
Cofilin
P
Chronophin
P
Cofilin
b
-Arrestin
actin
LIM
kinase
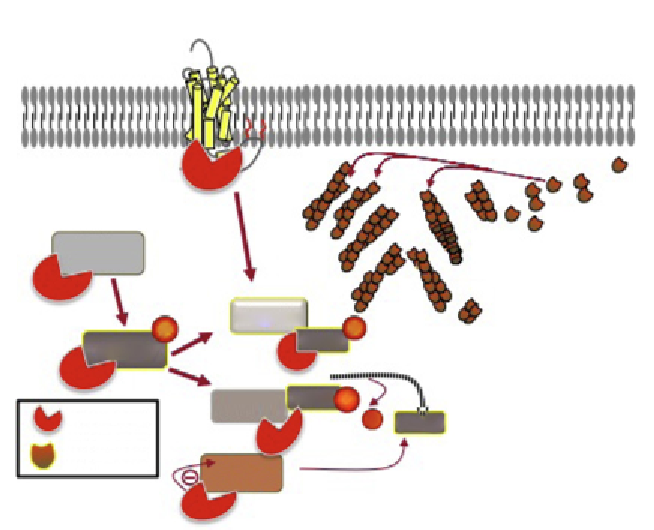
Figure 8.2 Model for regulation of cofilin activity by
b
-arrestins. Upon recruitment to
certain GPCRs,
b
-arrestins can activate cofilin through various mechanisms. First,
b
-arrestin-2 can directly bind the two cofilin phosphatases, slingshot and chronophin,
as well as cofilin. Association with
b
-arrestin-2 appears to scaffold cofilin to either sling-
shot or chronophin to facilitate dephosphorylation.
b
-Arrestin-1 can directly bind LIMK
and interfere with its phosphorylation of cofilin. Together, both
b
-arrestins can facilitate
GPCR-mediated cofilin dephosphorylation, which is essential for the creation of free bar-
bed ends at the leading edge for actin polymerization.
gradient. Thus, it is likely that
b
-arrestins contribute to the ability of a cell to
sense a gradient as well as form a leading edge through the regulation of
cofilin activity.
Reorganization of actin filaments by cofilin is important for other cellular
activities as well. In neurons, actin-rich dendritic spines form the postsynaptic
sites for most excitatory neurons and are important for normal learning and
memory processes. Dendritic spines are comprised primarily of filamentous
actin and dynamic reorganization of these structures is functionally linked
to synaptic plasticity. Many studies have demonstrated that spatially con-
strained cofilin activity is crucial to the dynamic nature of dendritic spines
and, subsequently, to synaptic plasticity. For example, the NMDA receptor
promotes remodeling of dendritic spines and translocation of active cofilin
Search WWH ::

Custom Search