Biology Reference
In-Depth Information
C
Agonist
Class A,
2
AR
P
P
GRKs
Stable receptor-
P
P
arrestin-Ub complex,
Endosome
Sustained pERK
on endosomes
-Arrestin
Ub
P
P
D
Agonist
P
Class B, V
2
R
P
P
P
P
GRKs
Transient receptor-
arrestin complex,
No pERK on endosomes
Endosome
No ubiquitination
-Arrestin-0K
-Arrestin-0K
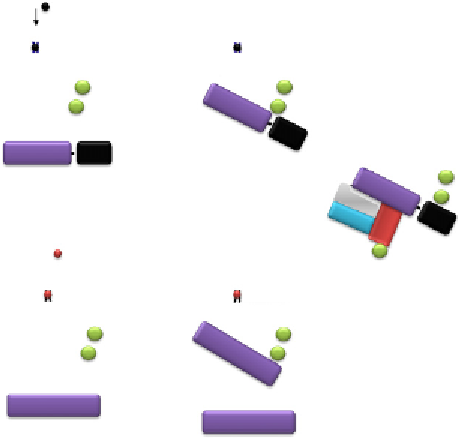
Figure 7.3 Cont'd (C) A b-arrestin2-Ub chimera, which cannot be deubiquitinated,
forms a stable complex with the class A b
2
AR and scaffolds active ERK on endosomes.
(D) The mutant b-arrestin2-0K that lacks ubiquitination sites forms a highly unstable
complex even with a class B receptor.
support the idea that the
b
-arrestin-binding pattern, as well as the stability
and signaling of receptor-
b
-arrestin complexes, is dictated by the molecular
signatures on the receptor carboxyl terminus as well as on
b
-arrestins,
namely, distinct phosphorylation motifs corresponding to either class A or
class B receptors, and ubiquitin chains on
b
-arrestin. The 7TMR recycling
and resensitization kinetics can be further dictated by ubiquitination of the
receptor itself.
Ubiquitination occurs on the
e
-amino group of lysine residues, and there
is no consensus sequence for substrate ubiquitination. Both
b
-arrestin
isoforms have many lysines (e.g., 35 in rat
b
-arrestin1 and 31 in rat
b
-arrestin2) dispersed along the length of the protein which presents a chal-
lenging task to identify specific lysines targeted for ubiquitination.
By undertaking series of mutagenesis steps, the lysine residues in
b
-arrestin2
targeted for stable ubiquitination as induced by the class B type AT
1a
R have
been mapped; here, the stable ubiquitination occurs primarily at the vicinal
lysines 11 and 12 in rat
b
-arrestin2.
56
Mutation of these lysines to arginines



















































































































Search WWH ::

Custom Search