Biology Reference
In-Depth Information
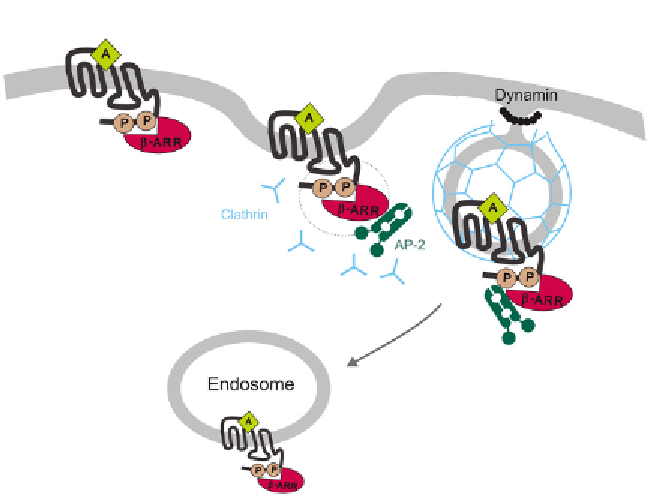
Figure 4.2 The prototypical model of b-arrestin-promoted sequestration of GPCRs via
the clathrin-mediated internalization pathway. Agonist-activated phosphorylated
receptors are guided to clathrin-coated pits by the recruitment of b-arrestin, which
binds to AP-2 and clathrin. The vesicles are subsequently pinched off by the GTPase
dynamin, which leads to receptor internalization into early endosomes.
more efficient at promoting its internalization.
75
In contrast, activation of
the angiotensin II type 1 receptor results in the recruitment of both
b
-arrestin-1 and -2, which display comparable efficiency in desensitizing
and internalizing the receptor.
34,75
These findings have led to the classifica-
tion of GPCRs into two subgroups that reflect the stability and outcome of
GPCR/arrestin interactions. Class A receptors, including
b
2AR,
m
-opioid
receptors, endothelin type A receptors, and dopamine D1A receptors,
have a higher affinity for
b
-arrestin-2 and form transient GPCR/arrestin
complexes that dissociate at/near the cell surface. In contrast, class
B receptors, such as V2 vasopressin, neurotensin 1, and angiotensin II type
1A receptors, bind
b
-arrestin-1 and -2 with equivalent affinity and form
stable GPCR/
b
-arrestin complexes that remain intact as the receptors
endocytose.
34,76,77
Chimeric receptors (
b
2AR/angiotensin II type 1 recep-
tors) have provided strong evidence that the stability of GPCR/
b
-arrestin
complexes are controlled by determinants within the GPCR carboxyl
terminal tail domains. Consequently, we now know that the receptor
Search WWH ::

Custom Search