Biomedical Engineering Reference
In-Depth Information
properties of the biomaterial that are important in
influencing these processes. A better understanding
of the influence biomaterials have on the infectious
process is expected to allow development of better,
more infection-resistant biomaterials; however, this is
an area that has been somewhat overlooked in the
past. This may be due in part to the fact that for the
majority of implanted biomaterials, the prevention or
treatment of infection is not a primary design concern.
For those implants where infection prevention is
a secondary design goal at best, the aim of product
development may be to ensure that susceptibility
to infection is not increased in comparison with
commercially available alternatives. This contrasts
with those implant types with a high risk of bacterial
colonization, such as urinary catheters and endotra-
cheal tubes or implants used to replace infected
material; for these implants, biomaterial design may
be driven by the need to offer greater protection from
bacterial colonization.
The biomaterial design strategies aimed at
achieving infection resistance fall into two broad
categories: first, surface modifications to minimize
bacterial adhesion and, second, antimicrobial incor-
poration into the biomaterial to kill any contami-
nating bacteria in the vicinity of the implant.
Minimizing bacterial adhesion to the biomaterial is
an obvious goal in biomaterial design. Once adhered
to a surface, the contaminating bacteria are known to
display increased resistance to host defenses and
antibiotic action in a manner that may be independent
of the specific properties of the biomaterial substrate.
Therefore, it is clearly preferable, and more achiev-
able, to prevent initial bacterial adhesion by bioma-
terial modification. A large number of biomaterial
modifications can be found in the literature, some of
which display greatly reduced adhesion; for example,
a 98% reduction was reported by Maddikeri et al.
[22]
. However, the clinical impact of such a reduction
on infection is still debatable and extremely hard to
quantify. This is primarily due to the fact that even
though bacterial adhesion may be reduced, even
a relatively low number of adherent bacteria can
multiply and grow to form a biofilm, ultimately
leading to infection. To achieve a greater impact
upon clinical infection rates, antimicrobial biomate-
rials have been developed. The delivery of active
antimicrobial agents into the surgical site, or at
the point of entry into the body, is expected to have
a much more significant impact on infection preven-
tion or in treatment than more passive strategies.
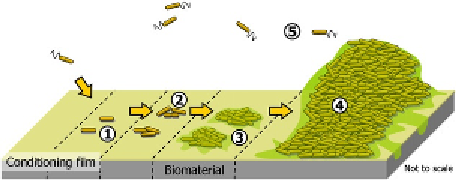
Figure 8.1
Diagram illustrating the main stages of
bacterial biofilm formation. (1) Nonspecific adhesion,
(2) specific adhesion, (3) proliferation and matrix
production, (4) maturation, and (5) dispersal. Adapted
from Stoodley et al.
[11]
.
and surgical interventions are illustrated in a clinical
study, where there was a doubling of surgical costs,
tripling of administrative costs, and a quadrupling of
ward costs for infected cases in comparison with
cases who did not develop any infection
[15]
. These
infections may manifest years after the original
implant surgery, leading to chronic problems such as
inflammation and osteomyelitis
[16,17]
. The risk of
infection is increased when the host tissue rejects and
encapsulates the biomaterial, forming a niche for
infection, which is isolated from the circulatory
system and unregulated by the immune system
[18]
.
For all the above reasons, it is, therefore, of the
upmost importance that modern biomaterials, such as
PEEK, are designed to reduce infection risk and
control the degree of tissue reaction.
8.1.2 Biomaterials and Infection
For many years, the biomaterial was considered to
be an innocent bystander in the infectious process,
whereby the combined effects of host defenses and
antibiotic administration are charged with the task of
clearing contaminating bacteria from the implant
site. It is becoming increasingly obvious, however,
that the biomaterial itself also plays a key role in the
development of an infection in two respects: first, by
providing a substrate upon which contaminating
bacteria may adhere to and form a biofilm
[19]
and,
second, by compromising the ability of the host cells
to kill bacteria in the vicinity of the implant
[20]
.It
was first shown in 1957 that the presence of an
implanted material increases the risk of infection
[21]
, when the presence of suture material was
shown to reduce the number of bacteria required to
cause an infection in human wounds 1000-fold. What
remains to be determined to date is the precise
reasoning for this and the key physical or chemical