Biomedical Engineering Reference
In-Depth Information
polymers are less crystalline than polymers with
more flexible backbones, such as polyethylene (PE)
and polypropylene (PP), and more crystalline than
materials with bulky side groups, such as polystyrene
(PS). In several studies, the crystallinity, morphology,
and associated thermal behavior of PEEK have been
compared with polyethylene terephthalate (PET),
a linear aromatic polymer that is connected via ester
linkages
[4
e
6]
.
PEEK can be up to about 40% crystalline
[7]
,
though 30
e
35% is more typical. The result is a two-
phase morphology, consisting of crystalline regions
dispersed in amorphous polymer. The two-phase
model has been successfully applied to predict and
describe the density of PEEK
[4]
, though some
researchers have also described a rigid amorphous
phase exhibiting precrystalline ordering that is
intermediate between the disordered amorphous
condition and the ordered array characteristic of the
crystalline phase
[8]
.
Organization develops as PEEK chains align in
a symmetric repeating manner that is described by the
unit cell of the crystal. The chains fold and pack to
form lamellae, which grow into three-dimensional
spherulites. The number and size of the spherulites
depend on the nucleation and growth processes and
affect the physical properties of the material. Crystal-
lization behavior is tightly linked to processing
conditions (i.e., thermal history), and some researchers
have suggested that this includes the development of
both positive and negative birefringences in PEEK
crystallites
[4,9]
.
(a)
(b)
(c)
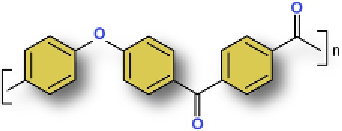
Figure 4.1
Schematic representation of polymer repeat
units for (a) PEEK, (b) PEK, (c) PEKK. Images courtesy of
Chris Espinosa, Exponent, Inc.
reported for PEEK and 34
for PEK
[3]
. Linkage
torsional angles can be affected by crystallization
temperature, with higher crystallization temperatures
resulting in more “twist” and less “stretch”
[3]
.
Large-scale structures are affected by the relative
polarity of the ether and ketone linkages and the twist
of the rings.
4.2.2 Morphology
PEEK and other PAEK polymers can be quenched
from the melt to form an amorphous, glassy solid. In
this state, the polymer chains adopt various confor-
mations that prevent efficient, regular packing, and
the material lacks long-range order. The amorphous
materials are generally transparent and exhibit lower
density than their crystalline counterparts. They also
lack the chemical resistance and mechanical prop-
erties that accompany the crystalline form. All
commercial polymers have some amorphous content
because polydispersity (i.e., a distribution of chain
lengths), the interconnected nature of the crystalliz-
able segments, and “defects” such as chain ends
prevent full crystallization.
Although it is possible to quench PAEK polymers
fast enough to create an amorphous, glassy material,
these polymers are readily crystallized either directly
during the manufacturing processes used to form
useful devices or during subsequent annealing
processes. Compared with other polymers, PAEK
4.2.3 Crystal Unit Cells
X-ray studies have confirmed that the unit cell of
PEEK is orthorhombic, exhibiting the symmetry of
space group Pcbn
[10]
. This unit cell and space group
applies to polyphenylene oxide (PPO), PEEK, and
PEK, which are linear aromatic polymers containing
100%, 66%, and 50% ether inter-ring linkages,
respectively. The unit cell of PEEK is depicted
schematically in
Fig. 4.2
. PEK also has an ortho-
rhombic unit cell
[2]
. In both PEEK and PEK, the
polymer backbone is aligned with the
c
-axis. The
structural repeating unit in crystals of the PAEK
polymers consists of two aryl rings. Chemically, the
repeating unit in PEEK contains three aryl rings,
which for symmetry expands to six aryl rings
[3]
. The
crystallographic pseudo repeat unit
is shown in
Fig. 4.3
.