Biomedical Engineering Reference
In-Depth Information
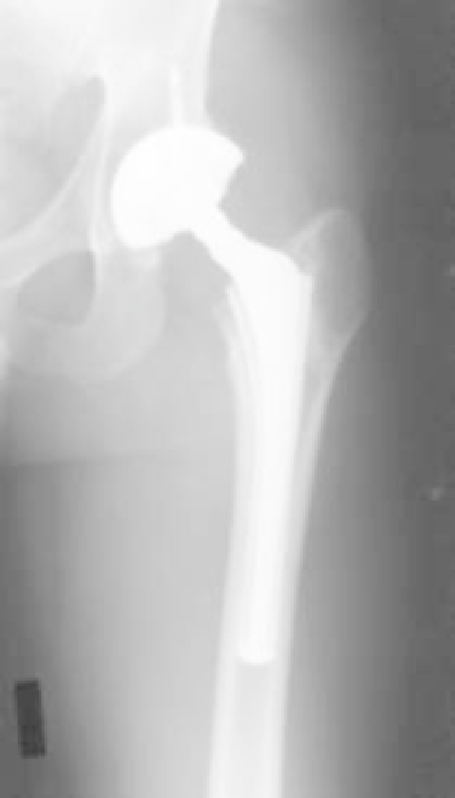
(a)
(c)
(b)
Figure 14.3
Continued.
considered by Zimmer, but engineers observed
polymer crazing of the composite hip stems upon
exposure to a simulated in vivo environment con-
taining lipids (
Fig. 14.5
). Challenges in material
selection, biocompatibility evaluation, and manu-
facturability were considered in parallel. Because
PAEK polymers were novel to orthopedics, a broad
range of biocompatibility tests were performed,
including cytotoxicity, carcinogenicity, acute
systemic toxicity, genotoxicity (ames mutagenicity),
genotoxicity (mouse lymphoma mutagenicity),
hemocompatibility, intracutaneous reactivity, mate-
rial-mediated pyrogenic response, and sensitization
[35]
. PAEK polymers did not exhibit an adverse
response when evaluated in this broad range of
biocompatibility tests.
The original Epoch stem (currently referred to as
“Epoch I”) was a three-part macrocomposite con-
sisting of a forged CoCr alloy inner core, an inter-
mediate layer of PEKEKK resin (UltraPek: BASF
Corporation), and outer bone ingrowth layer of
commercially pure Ti fiber mesh (
Fig. 14.6
).
A complex multistage manufacturing process had to
be developed for the Epoch stem. The CoCr core and
Ti fiber metal mesh were first fabricated and assem-
bled (
Fig. 14.7a
). The core/mesh assembly was
loaded into an injection-molding press, and molten
Figure 14.3
(a) First and second generations of the
Epoch hip stem (Zimmer, Warsaw IN). The Epoch I
stem was an anatomic design for left or right hips
and was fabricated from PEKEKK. The Epoch II design
is a straight stem and is fabricated from PEEK. (b) Ante-
rioreposterior radiograph of Epoch Stem at 10 years
follow-up. (c) Postoperative lateral radiograph of Epoch
stem.