Biomedical Engineering Reference
In-Depth Information
In recent years, there has been great interest in
finding ways to halt the degenerative cascade
following fusion surgery
[9,10]
. Because fusion
results in unnatural motion at adjacent levels,
researchers have proposed that preserving normal
motion in the spine will help alleviate, and perhaps
even prevent, adjacent segment degeneration.
Consequently, a variety of new implant technologies
have developed to preserve, limit, or enhance motion
of the spine. Although previous fusion technologies
have been referred to as “static” or rigid fusion, new
fusion technologies that employ a more flexible
instrumentation systems are gaining clinical accep-
tance. Interspinous implants, such as the X-STOP
(Kyphon, Sunnyvale, CA), have been developed with
PEEK-OPTIMA components to treat back pain
caused by spinal stenosis
[11]
. Artificial discs,
developed with PEEK components, represent another
novel implant technology that is intended to preserve
motion of the treated spine
[12]
.
Motion preservation spine technology, while
perhaps no longer in its infancy, still remains in the
very early years of development and clinical accep-
tance. In 2008, an estimated 5000 interspinous
process implants, 800 posterior pedicle-based stabi-
lization devices, and 4900 total disc replacements
(TDRs, including both cervical and lumbar) were
performed in the United States based on data from
the NIS. Reimbursement for surgeons and hospitals
for performing motion preserving spinal surgery
continues to be extremely challenging in the United
States. Thus, motion preservation should be viewed
through the lens of an early development in the spine
field; fusion procedures currently dominate the clin-
ical practice of spine surgery.
This chapter focuses on the variety of spinal
implant applications of PEEK. We begin with an
overview of interbody fusion and historical devel-
opment of the first carbon fiber-reinforced (CFR)-
PEEK spinal implant, the Brantigan I/F lumbar
fusion cage. More recent applications of PEEK in the
spine, including dynamic stabilization devices and
artificial discs, are also reviewed.
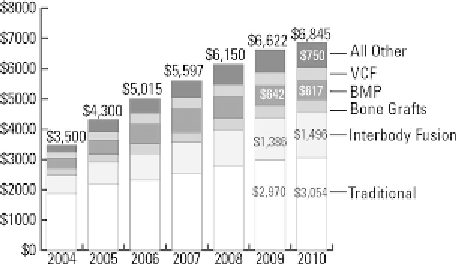
Figure 13.2
2004
e
2010 spinal implant US sales
($ millions) by segment. Reproduced with permission
from the
Orthopedic Network News
[6]
.
to 1.5 billion US$ in 2010 (
Fig. 13.2
)
[6]
. The posi-
tioning of PEEK cages within the interbody market
has likewise expanded since Food and Drug
Administration (FDA) approval in 2001. By 2010,
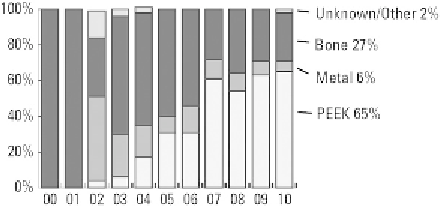
PEEK cages accounted for 65% of interbody devices,
representing a market of approximately 1 billion US$
in the United States alone (
Fig. 13.3
)
[6]
. PEEK
cages have also contributed to the growth of the
$613m recombinant human bone morphogenetic
protein-2 (BMP) market (
Fig. 13.2
). The use of BMP
for facilitating interbody fusion was approved by the
FDA in 2002 for the threaded titanium LT-Cage
[7]
,
but it has been increasingly used “off label” with
other spinal fusion devices
[8]
. Metal cages, such as
the LT-Cage fabricated from titanium, are estimated
to constitute 10% of the interbody market (
Fig. 13.3
)
[6]
. Thus, the vast majority of BMP use for interbody
fusion is accomplished using PEEK rather than metal
cages. Compared with other synthetic biomaterial
solutions, PEEK biomaterials now dominate the
design space for interbody cages and are the principal
implant delivery system for BMP.
13.2 Origins of Interbody Fusion
and the “Cage Rage” of the Late
1990s
Figure 13.3
Trends in materials used in interbody
fusion devices, 2000
Although lumbar and cervical fusions are today
considered
2010. Reproduced with permis-
sion from the
Orthopedic Network News
[6]
.
e
common
procedures,
the
surgical