Biomedical Engineering Reference
In-Depth Information
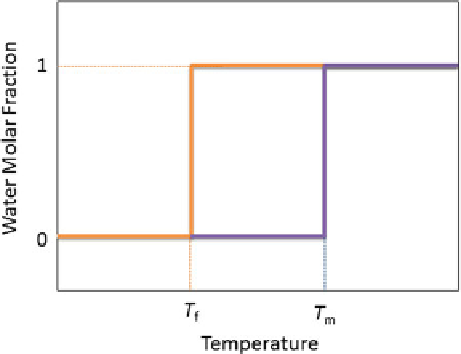
Fig. 2.1
Definition of
hysteresis (
D
T
m
T
f
)
nucleation will be reviewed. The inhibition of AFP and AFGPs on the growth of
ice crystals will also be discussed on a surface structural analysis. We notice that
although there have been a significant number of papers on AFPs, a comprehensive
overview on freezing promotion and antifreeze mechanisms from a kinetic point of
view has never been achieved yet. The applications of AFP and AFGPs will also
be reviewed at the end. This chapter will provide a platform to review the works in
this area. Hopefully, the knowledge obtained here will provide some basic ideas to
mimic the antifreeze effect without AFPs.
2.1.2
Thermal Hysteresis of AFPs
There are two classes of substances that inhibit water freezing. The first class
comprises those solutes that depress both freezing point and melting point. Such
substances include sodium chloride, glycerol, glucose, etc. The first class is to
change the equilibrium point and can be regarded as a thermodynamic approach.
The second class comprises substances having the ability to depress freezing point
without significantly affecting the melting point. This is attributed to the change
of the freezing kinetics, mainly nucleation and growth. Therefore, the substances
mainly block ice formation based on the kinetic factors.
AFPs, also known as thermal hysteresis proteins, lowering the nonequilibrium
freezing point of water while not significantly affecting the melting point, belong to
the second class. The difference between the freezing and melting points is termed
“thermal hysteresis.” The magnitude of this characteristic thermal hysteresis activity
is dependent upon the specific activity and concentration of the particular AFP [
25
]
(Fig.
2.1
).