Biomedical Engineering Reference
In-Depth Information
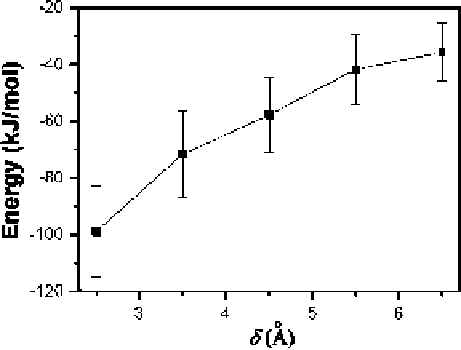
Fig. 1.35
The electrostatic
interaction energies between
the external charge and the
water molecules with respect
to the distance of the external
charge from the tube wall
(defined by ı) for the case of
System I when
q
DC
1
e
.The
error bars
show the
fluctuations due to thermal
noise (reprinted from [
42
].
Copyright 2009 American
Chemical Society)
for a successful manipulation may even be higher for a much lower speed under
experimental conditions. When the peptide is substituted by a larger molecule
such as a protein, the effective value of the external charge required for successful
manipulation should be larger. We note that in the case where the probability of the
successful manipulations by a single charge is low, we can use a series of charges,
which can greatly enhance the successful probabilities.
Third, the distance of the external charge from the tube wall (defined by ı)also
influences the manipulation. For System I, as is shown in Fig.
1.35
, the electrostatic
interaction energy between the external charge with the effective value of
q
DC
1
e
and the water molecules in the peptide-water mixture increases gradually as ı
increases. Numerically, our simulation shows that even when ı is up to 6.5 A, the
peptide-water mixture can also follow the external charge well. When
q
DC
0.5
e
,
we have observed two of the three cases with different initial conditions that the
peptide-water mixture follows the external charge in 12 ns simulation when the
distance is 5.5 A, with an electrostatic interaction energy between the external
charge and the water molecules of
10.5
˙
6.3 kJ/mol, obtained from the successful
manipulation cases.
Finally, since our simulations are based on the stochastic dynamics, the damping
coefficient used will influence the manipulation. For System I, when the damping
coefficient
D
0.01 ps
1
, according to the Langevin equation, the force due to
Langevin damping is
f
damping
D
M
v
D
0.153 pN, in which
M
is the mass of peptide-
water mixture, if we assume that the peptide-water mixture follows the external
charge extremely well with the same velocity
v
D
1 m/s. This value of
f
damping
is
much smaller than the electrostatic force that the external charge exerts on the water
molecules. We find that some modifications of the value of do not change the
results much in this chapter. Our calculation shows that when
D
0.1 ps
1
,the
electrostatic interaction energy is
17.0
˙
8.5 kJ/mol (
q
DC
0.5
e
), which is very
close to the value of
18.5
˙
7.9 kJ/mol for
D
0.01 ps
1
case; moreover, the
peptide-water mixture still follows the external charge well in the 12 ns simulation