Biomedical Engineering Reference
In-Depth Information
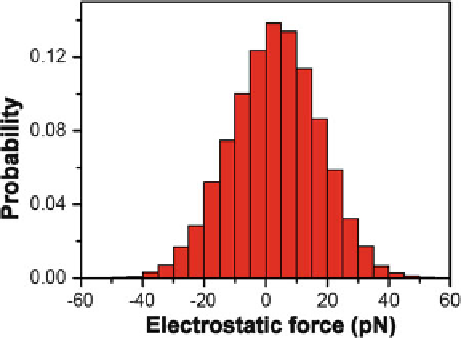
Fig. 1.33
The electrostatic
forces by the external charge
exerting on the peptide-water
mixture along the
x
-axis for
the case of System I when
q
0.5
e
. The positive
direction of the force is the
positive direction of the
x
-axis,anda5pNforce
interval is used (reprinted
from [
42
]. Copyright 2009
American Chemical Society)
DC
nanoscale pores can lead to interesting properties and behaviors that significantly
differ from those of bulk systems [
6
,
21
,
22
,
32
,
40
,
43
-
59
], including the enhanced
catalysis [
44
,
45
] and enhanced stability of the native structure of proteins [
47
],
new folding mechanisms of proteins [
49
,
50
], ordered water structure [
52
-
54
],
extrafast motion of water molecules [
6
,
21
,
46
], non-Fickian-type diffusion [
55
],
and excellent on-off gating behavior [
22
,
40
]. Furthermore, it has been found
that when the molecules are confined in nanosized water droplets [
60
,
61
], their
structures, hydrophobic and ionic interactions differ from those in bulk water [
62
,
63
]. Manipulating the positions of the molecules encapsulated in the nanopores with
respect to time is important in controlling the interactions or chemical reactions
of the inner molecules. In recent years, there have been considerable efforts
[
14
,
64
-
70
] devoted to the study of the translocation/permeation of molecules
along/through the nanochannels. Yeh and Hummer used an electric field to drive
the charged macromolecules through nanopores [
64
]. Longhurst and Quirke made
use of capillary force to draw decane molecules into an SWNT and temperature
difference to drive their transport through the SWNT [
67
]. Zhao et al. demonstrated
experimentally that a water flow can be driven by the applied current of the SWNT
[
14
]. Kral used laser to excitate an electric current in the CNT, thus resulting in a
net force on ions absorbed in the nanotube [
70
].
We have also calculated the electrostatic force that the external charge exerts on
the peptide-water mixture along the
x
-axis. The electrostatic forces dominatively
range from
40 to
C
40 pN (see Fig.
1.33
), which fall within the working ranges
of many existing techniques such as STM and AFM. This result suggests that
the AFM/STM tip carrying charge(s) may be able to manipulate the peptide with
aqueous liquids according to the method described here.
Furthermore, on the basis of the above design, we can controllably move two
biomolecule-water mixtures together conveniently for the interaction of the two
biomolecules, as demonstrated in Fig.
1.34
. Here, we use the same peptides used