Biomedical Engineering Reference
In-Depth Information
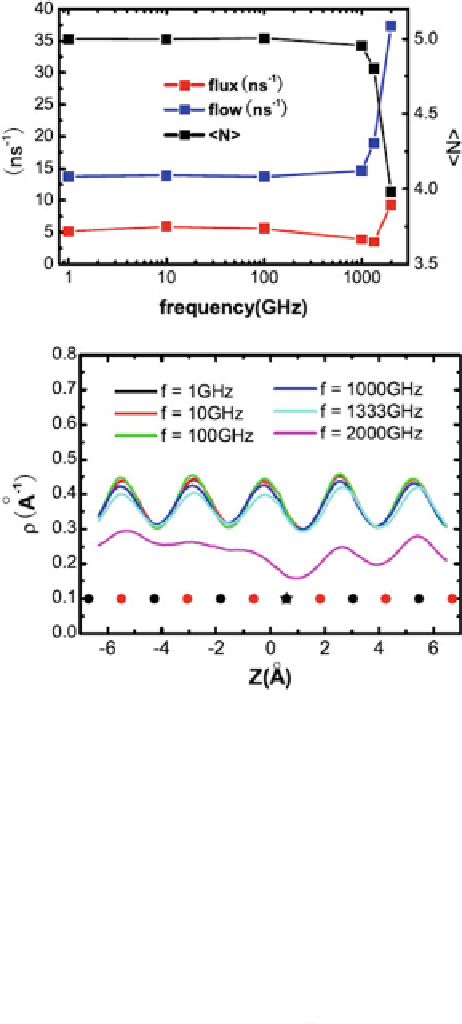
Fig. 1.20
Flux, flow, and
average number of water
molecules inside the CNT for
different vibrating
frequencies
f
(reprinted from
[
130
]. Copyright 2008
Chinese Physics Society)
Fig. 1.21
Water density
distribution along the
nanotube axis. The
open
and
filled circles
denote the
locations of the carbon atoms.
The
asterisk
is the position of
the vibrating atom affected by
an external force (reprinted
from [
130
]. Copyright 2008
Chinese Physics Society)
Fluctuations of CNT induce the number of water molecules inside the nanotube to
decrease and the velocity of the transportation of water chain to increase.
Water molecules confined inside the (6,6) CNT form a single-filed chain
connected by hydrogen bonds. An important feature of the hydrogen bond is that
it possesses directionality (see Fig.
1.1
). Molecular transport through the quasi-one-
dimensional CNT is highly collective, since motion of one water molecule requires
concomitant motion of all water molecules in the file. The chain rarely ruptures
because of the tight hydrogen bonds in the protective environment of the CNT.
Hydrogen bonds nearly align along the nanochannel axis and collectively flip in
th
eir orientations. The orientation of water
ch
ain is defined by a ch
ar
acteristic ang
le
' [
40
]. There are two stable states 15
ı
< '<50
ı
and 130
ı
< ' < 165
ı
and '
switches between them (see Fig.
1.22
). Her
e
we define a flip as ' transforms from
one state to another state passing through '
D
90
ı
. During the flipping process,
under the transition state, a hydrogen bond defect along the water chain inside the
SWNT is formed. The flipping frequency f
flip
for different vibrating frequency is
shown in Fig.
1.23
. Interestingly, the effect of the vibration of the CNT on the