Biomedical Engineering Reference
In-Depth Information
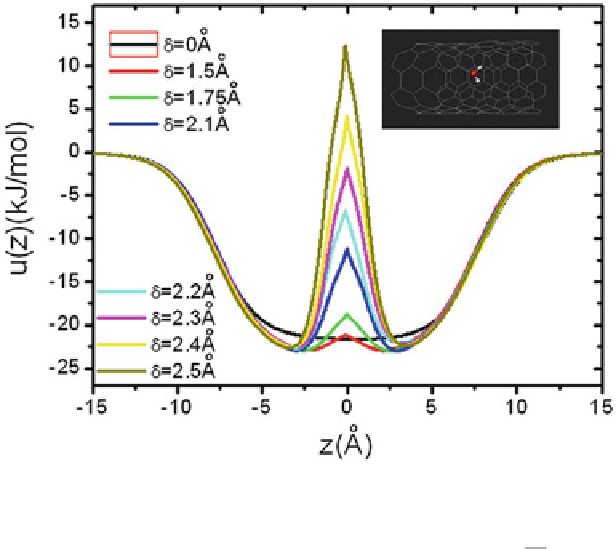
Fig. 1.17
Water-CNT interaction energy
u
(
z
) of a water molecule at
z
with the carbon nanotube.
The inset shows a water molecule locating in the center of the cross section at
z
of the carbon
nanotube. Water-CNT interaction,
u
(
z
), is calculated by
u
.
z
/ D 4"
CO
6
,
where
r
i
is the distance between the water molecule and
i
th carbon atom. In the calculation, we
assume that the water molecule locates at the center of the cross section of the nanotube (reprinted
from [
129
]. Copyright 2008 American Physical Society)
CO
r
i
12
CO
r
i
i D156
i D1
Now, we present a theoretical model to understand the origin of the wavelike
pattern of water density along the nanotube axis. There are three assumptions:
1. Water molecules inside the nanotube form a single-filed chain.
2. The distance between any two neighboring water molecules inside the nanotube
along the
z
direction is fixed, which is denoted by
d
.
3. The change in free energy of the single-filed water inside the nanotube mainly
results from the change in water-CNT interaction energy
u
(
z
) when the CNT
is deformed by the external force. Water molecules inside the nanotube form a
single-filed chain.
If we know the water-CNT interaction energy
u
(
z
) and the distance between
the two neighboring water molecules inside the CNT, according to the above
three assumptions, we can calculate the water probability density along the CNT.
Both the water-CNT interaction and the distance between the two neighboring
water molecules can be obtained without numerical simulations.
u
(
z
)isshownin
Fig.
1.17
. The equilibrium distance between the two water molecules is about 2.8 A.
Considering that the water molecules inside the channel are usually connected by