Biomedical Engineering Reference
In-Depth Information
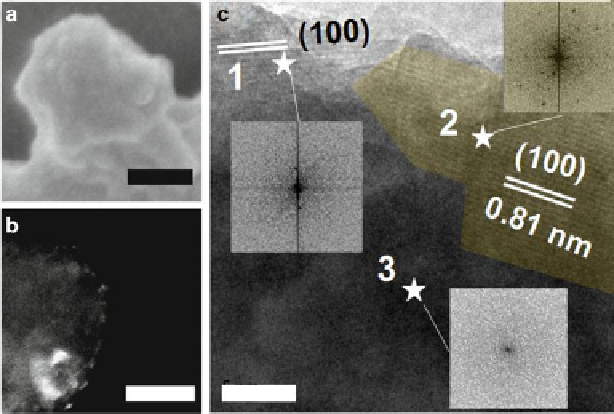
Fig. 7.9
Initial phase transformation stage of ACP particles. (
a
)SEMimage(
b
) DF-TEM image.
(
c
) HR-TEM image, and the fast Fourier transform (
FFT
) patterns of crystallized regions (
1
,
2
)
and the amorphous region (
3
). Bar: (
a
) 100 nm; (
b
)50nm;(
c
) 10 nm. Reproduced with permission
from Ref. [
63
] © The Royal Society of Chemistry 2010
The above results indicate that at the initial stage of biomineralization in a
simulated body fluid solution, ACP spheres occur soon after mixing the two
reactants. The transformation from ACP to HAP takes place in about 1 h. During the
transformation, the nucleation occurs preferably at the surface of ACP spheres. The
embedded/adhered crystallites on the ACP surface would not allow the crystallites
to rotate their orientations and/or relocate from their relative positions. This gives
rise to the formation of the HAP spherulites.
The process outlined above is a typical stepwise crystallization, the so-called
two-step crystallization (TSC) [
66
]. It is one way to facilitate the nucleation kinetics
and often observed during protein crystallization [
10
,
67
-
69
], biomineralization,
etc. According to TSC, dense amorphous droplets are first formed from the mother
phase; crystalline nuclei are then created from the droplets. For instance, during the
formation of calcite in sea urchin larvae, a transient amorphous phase is formed first,
before the final crystal phase is reached [
70
,
71
]. Similarly, a transient amorphous
phase is also identified during the formation of aragonite controlled by mollusk
bivalve larvae [
72
]. Recently, the similar process has also been observed for the HAP
formation from a simulated body fluid [
73
]. It is widely believed that in biological
systems, the development of crystalline structures characterized by well-defined
shape and size is essentially facilitated by the occurrence of transient amorphous
phases [
64
,
71
,
72
]. In fact, recent studies indicated that TSC may be a mechanism
underlying most crystallization occurring in typical atomic systems [
62
,
73
,
74
].